Stereoselective Fluorescence Quenching in the Electron Transfer Photooxidation of Nucleobase-Related Azetidines by Cyanoaromatics
- PMID: 27941606
- PMCID: PMC6273614
- DOI: 10.3390/molecules21121683
Stereoselective Fluorescence Quenching in the Electron Transfer Photooxidation of Nucleobase-Related Azetidines by Cyanoaromatics
Abstract
Electron transfer involving nucleic acids and their derivatives is an important field in bioorganic chemistry, specifically in connection with its role in the photo-driven DNA damage and repair. Four-membered ring heterocyclic oxetanes and azetidines have been claimed to be the intermediates involved in the repair of DNA (6-4) photoproduct by photolyase. In this context, we examine here the redox properties of the two azetidine isomers obtained from photocycloaddition between 6-aza-1,3-dimethyluracil and cyclohexene. Steady-state and time-resolved fluorescence experiments using a series of photoreductants and photooxidants have been run to evaluate the efficiency of the electron transfer process. Analysis of the obtained quenching kinetics shows that the azetidine compounds can act as electron donors. Additionally, it appears that the cis isomer is more easily oxidized than its trans counterpart. This result is in agreement with electrochemical studies performed on both azetidine derivatives.
Keywords: DNA repair; energy and charge transfer; nucleobase analogues; photolyase; redox potential.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
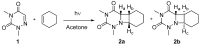




Similar articles
-
Theoretical Study on the Photo-Oxidation and Photoreduction of an Azetidine Derivative as a Model of DNA Repair.Molecules. 2021 May 14;26(10):2911. doi: 10.3390/molecules26102911. Molecules. 2021. PMID: 34068908 Free PMC article.
-
Experimental and Theoretical Study on the Cycloreversion of a Nucleobase-Derived Azetidine by Photoinduced Electron Transfer.Chemistry. 2018 Oct 12;24(57):15346-15354. doi: 10.1002/chem.201803298. Epub 2018 Sep 11. Chemistry. 2018. PMID: 30053323
-
Cycloreversion of azetidines via oxidative electron transfer. steady-state and time-resolved studies.Org Lett. 2008 Nov 20;10(22):5207-10. doi: 10.1021/ol802181u. Epub 2008 Oct 21. Org Lett. 2008. PMID: 18937481
-
Trans-diammineplatinum(II): what makes it different from cis-DDP? Coordination chemistry of a neglected relative of cisplatin and its interaction with nucleic acids.Met Ions Biol Syst. 1996;33:105-41. Met Ions Biol Syst. 1996. PMID: 8742842 Review.
-
Ring-opening of azetidiniums by nucleophiles. Synthesis of polysubstituted linear amines.Chirality. 2021 Jan;33(1):5-21. doi: 10.1002/chir.23280. Epub 2020 Nov 17. Chirality. 2021. PMID: 33201588 Review.
Cited by
-
Photorelaxation and Photorepair Processes in Nucleic and Amino Acid Derivatives.Molecules. 2017 Dec 12;22(12):2203. doi: 10.3390/molecules22122203. Molecules. 2017. PMID: 29231852 Free PMC article.
-
Theoretical Study on the Photo-Oxidation and Photoreduction of an Azetidine Derivative as a Model of DNA Repair.Molecules. 2021 May 14;26(10):2911. doi: 10.3390/molecules26102911. Molecules. 2021. PMID: 34068908 Free PMC article.
-
Synthesis of azetidines by aza Paternò-Büchi reactions.Chem Sci. 2020 Jun 12;11(29):7553-7561. doi: 10.1039/d0sc01017k. eCollection 2020 Aug 7. Chem Sci. 2020. PMID: 32832061 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

