Investigating Sterol and Redox Regulation of the Ion Channel Activity of CLIC1 Using Tethered Bilayer Membranes
- PMID: 27941637
- PMCID: PMC5192407
- DOI: 10.3390/membranes6040051
Investigating Sterol and Redox Regulation of the Ion Channel Activity of CLIC1 Using Tethered Bilayer Membranes
Abstract
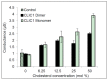
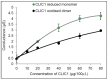
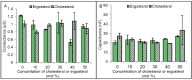
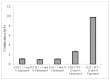
The Chloride Intracellular Ion Channel (CLIC) family consists of six conserved proteins in humans. These are a group of enigmatic proteins, which adopt both a soluble and membrane bound form. CLIC1 was found to be a metamorphic protein, where under specific environmental triggers it adopts more than one stable reversible soluble structural conformation. CLIC1 was found to spontaneously insert into cell membranes and form chloride ion channels. However, factors that control the structural transition of CLIC1 from being an aqueous soluble protein into a membrane bound protein have yet to be adequately described. Using tethered bilayer lipid membranes and electrical impedance spectroscopy system, herein we demonstrate that CLIC1 ion channel activity is dependent on the type and concentration of sterols in bilayer membranes. These findings suggest that membrane sterols play an essential role in CLIC1's acrobatic switching from a globular soluble form to an integral membrane form, promoting greater ion channel conductance in membranes. What remains unclear is the precise nature of this regulation involving membrane sterols and ultimately determining CLIC1's membrane structure and function as an ion channel. Furthermore, our impedance spectroscopy results obtained using CLIC1 mutants, suggest that the residue Cys24 is not essential for CLIC1's ion channel function. However Cys24 does appear important for optimal ion channel activity. We also observe differences in conductance between CLIC1 reduced and oxidized forms when added to our tethered membranes. Therefore, we conclude that both membrane sterols and redox play a role in the ion channel activity of CLIC1.
Keywords: CLIC; chloride intracellular ion channel proteins; cholesterol; ergosterol; tethered lipid membranes.
Conflict of interest statement
Bruce A. Cornell is employed by Surgical Diagnostics Pty Ltd.
Figures



References
-
- Littler D.R., Harrop S.J., Goodchild S.C., Phang J.M., Mynott A.V., Jiang L., Valenzuela S.M., Mazzanti M., Brown L.J., Breit S.N., et al. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010;584:2093–2101. doi: 10.1016/j.febslet.2010.01.027. - DOI - PubMed
-
- Valenzuela S.M., Alkhamici H., Brown L.J., Almond O.C., Goodchild S.C., Carne S., Curmi P.M., Holt S.A., Cornell B.A. Regulation of the membrane insertion and conductance activity of the metamorphic chloride intracellular channel protein CLIC1 by cholesterol. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0056948. - DOI - PMC - PubMed
-
- Warton K., Tonini R., Fairlie W.D., Matthews J.M., Valenzuela S.M., Qiu M.R., Wu W.M., Pankhurst S., Bauskin A.R., Harrop S.J. Recombinant CLIC1 (NCC27) assembles in lipid bilayers via a ph-dependent two-state process to form chloride ion channels with identical characteristics to those observed in chinese hamster ovary cells expressing CLIC1. J. Biol. Chem. 2002;277:26003–26011. doi: 10.1074/jbc.M203666200. - DOI - PubMed
-
- Littler D.R., Harrop S.J., Fairlie W.D., Brown L.J., Pankhurst G.J., Pankhurst S., DeMaere M.Z., Campbell T.J., Bauskin A.R., Tonini R. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J. Biol. Chem. 2004;279:9298–9305. doi: 10.1074/jbc.M308444200. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

