Non-Canonical Cell Death Induced by p53
- PMID: 27941671
- PMCID: PMC5187868
- DOI: 10.3390/ijms17122068
Non-Canonical Cell Death Induced by p53
Abstract
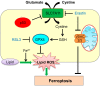
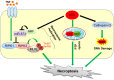
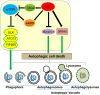
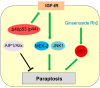
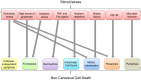
Programmed cell death is a vital biological process for multicellular organisms to maintain cellular homeostasis, which is regulated in a complex manner. Over the past several years, apart from apoptosis, which is the principal mechanism of caspase-dependent cell death, research on non-apoptotic forms of programmed cell death has gained momentum. p53 is a well characterized tumor suppressor that controls cell proliferation and apoptosis and has also been linked to non-apoptotic, non-canonical cell death mechanisms. p53 impacts these non-canonical forms of cell death through transcriptional regulation of its downstream targets, as well as direct interactions with key players involved in these mechanisms, in a cell type- or tissue context-dependent manner. In this review article, we summarize and discuss the involvement of p53 in several non-canonical modes of cell death, including caspase-independent apoptosis (CIA), ferroptosis, necroptosis, autophagic cell death, mitotic catastrophe, paraptosis, and pyroptosis, as well as its role in efferocytosis which is the process of clearing dead or dying cells.
Keywords: apoptosis; autophagy; caspase-independent apoptosis (CIA); efferocytosis; ferroptosis; mitotic catastrophe; necroptosis; paraptosis; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Using Small Molecules to Dissect Non-apoptotic Programmed Cell Death: Necroptosis, Ferroptosis, and Pyroptosis.Chembiochem. 2015 Dec;16(18):2557-61. doi: 10.1002/cbic.201500422. Epub 2015 Oct 16. Chembiochem. 2015. PMID: 26388514 Review.
-
p53-regulated non-apoptotic cell death pathways and their relevance in cancer and other diseases.Nat Rev Mol Cell Biol. 2025 Aug;26(8):600-614. doi: 10.1038/s41580-025-00842-3. Epub 2025 Apr 9. Nat Rev Mol Cell Biol. 2025. PMID: 40204927 Review.
-
The Crosstalk Between Long Non-Coding RNAs and Various Types of Death in Cancer Cells.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211033044. doi: 10.1177/15330338211033044. Technol Cancer Res Treat. 2021. PMID: 34278852 Free PMC article. Review.
-
Cell death: a review of the major forms of apoptosis, necrosis and autophagy.Cell Biol Int. 2019 Jun;43(6):582-592. doi: 10.1002/cbin.11137. Epub 2019 Apr 25. Cell Biol Int. 2019. PMID: 30958602 Review.
-
Mechanical insights into the regulation of programmed cell death by p53 via mitochondria.Biochim Biophys Acta Mol Cell Res. 2019 May;1866(5):839-848. doi: 10.1016/j.bbamcr.2019.02.009. Epub 2019 Feb 18. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30790591 Review.
Cited by
-
Mitochondrial sirtuin 3 and various cell death modalities.Front Cell Dev Biol. 2022 Jul 22;10:947357. doi: 10.3389/fcell.2022.947357. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35938164 Free PMC article. Review.
-
Hexavalent chromium intoxication induces intrinsic and extrinsic apoptosis in human renal cells.Mol Med Rep. 2020 Feb;21(2):851-857. doi: 10.3892/mmr.2019.10885. Epub 2019 Dec 16. Mol Med Rep. 2020. PMID: 31974625 Free PMC article.
-
Photoreceptor Degeneration in Pro23His Transgenic Rats (Line 3) Involves Autophagic and Necroptotic Mechanisms.Front Neurosci. 2020 Nov 3;14:581579. doi: 10.3389/fnins.2020.581579. eCollection 2020. Front Neurosci. 2020. PMID: 33224023 Free PMC article.
-
Integrative p53, micro-RNA and Cathepsin Protease Co-Regulatory Expression Networks in Cancer.Cancers (Basel). 2020 Nov 20;12(11):3454. doi: 10.3390/cancers12113454. Cancers (Basel). 2020. PMID: 33233599 Free PMC article. Review.
-
Activated p53 in the anti-apoptotic milieu of tuberous sclerosis gene mutation induced diseases leads to cell death if thioredoxin reductase is inhibited.Apoptosis. 2021 Jun;26(5-6):253-260. doi: 10.1007/s10495-021-01670-4. Epub 2021 Apr 16. Apoptosis. 2021. PMID: 33860865 Free PMC article.
References
-
- Vogt C. Untersuchungen Uber Die Entwicklungsgeschichte der Geburtshelferkröte (Alytes Obstetricans) Jent und Gassmann; Solothurn, Switzerland: 1984.
-
- Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R., et al. Classification of cell death: Recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

