Influence of Tyrosine Kinase Inhibitors on Hypertension and Nephrotoxicity in Metastatic Renal Cell Cancer Patients
- PMID: 27941701
- PMCID: PMC5187873
- DOI: 10.3390/ijms17122073
Influence of Tyrosine Kinase Inhibitors on Hypertension and Nephrotoxicity in Metastatic Renal Cell Cancer Patients
Abstract
Renal cell carcinoma (RCC) is one of the most common kidney malignancies. An upgraded comprehension of the molecular biology implicated in the development of cancer has stimulated an increase in research and development of innovative antitumor therapies. The aim of the study was to analyze the medical literature for hypertension and renal toxicities as the adverse events of the vascular endothelial growth factor (VEGF) signaling pathway inhibitor (anti-VEGF) therapy. Relevant studies were identified in PubMed and ClinicalTrials.gov databases. Eligible studies were phase III and IV prospective clinical trials, meta-analyses and retrospective studies that had described events of hypertension or nephrotoxicity for patients who received anti-VEGF therapy. A total of 48 studies were included in the systematic review. The incidence of any grade hypertension ranged from 17% to 49.6%. Proteinuria and increased creatinine levels were ascertained in 8% to 73% and 5% to 65.6% of patients, respectively. These adverse events are most often mild in severity but may sometimes lead to treatment discontinuation. Nephrotoxicity and hypertension are related to multiple mechanisms; however, one of the main disturbances in those patients is VEGF inhibition. There is a significant risk of developing hypertension and renal dysfunction among patients receiving anti-VEGF treatment; however, there is also some evidence that these side effects may be used as biomarkers of response to antiangiogenic agents.
Keywords: hypertension; nephrotoxicity; renal cell carcinoma; tyrosine kinase inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
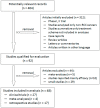
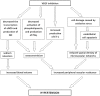
References
-
- GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. [(accessed on 11 July 2016)]. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
-
- Common Terminology Criteria for Adverse Events (CTCAE) V 3.0. [(accessed on 10 June 2016)]; Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/....
-
- Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Oudard S., Negrier S., Szczylik C., Pili R., Bjarnason G.A., et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

