Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease
- PMID: 27941791
- PMCID: PMC5405698
- DOI: 10.1038/nm.4242
Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease
Abstract
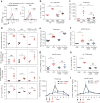
Excess levels of protein in urine (proteinuria) is a hallmark of kidney disease that typically occurs in conjunction with diabetes, hypertension, gene mutations, toxins or infections but may also be of unknown cause (idiopathic). Systemic soluble urokinase plasminogen activator receptor (suPAR) is a circulating factor implicated in the onset and progression of chronic kidney disease (CKD), such as focal segmental glomerulosclerosis (FSGS). The cellular source(s) of elevated suPAR associated with future and progressing kidney disease is unclear, but is likely extra-renal, as the pathological uPAR is circulating and FSGS can recur even after a damaged kidney is replaced with a healthy donor organ. Here we report that bone marrow (BM) Gr-1lo immature myeloid cells are responsible for the elevated, pathological levels of suPAR, as evidenced by BM chimera and BM ablation and cell transfer studies. A marked increase of Gr-1lo myeloid cells was commonly found in the BM of proteinuric animals having high suPAR, and these cells efficiently transmit proteinuria when transferred to healthy mice. In accordance with the results seen in suPAR-associated proteinuric animal models, in which kidney damage is caused not by local podocyte-selective injury but more likely by systemic insults, a humanized xenograft model of FSGS resulted in an expansion of Gr-1lo cells in the BM, leading to high plasma suPAR and proteinuric kidney disease. Together, these results identify suPAR as a functional connection between the BM and the kidney, and they implicate BM immature myeloid cells as a key contributor to glomerular dysfunction.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures


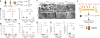
Comment in
-
A bone marrow factor contributes to kidney disease.Nat Med. 2017 Jan 6;23(1):13-14. doi: 10.1038/nm.4263. Nat Med. 2017. PMID: 28060796 No abstract available.
-
Glomerular disease: A suPAR kidney connection found in the bone marrow.Nat Rev Nephrol. 2017 May;13(5):263-264. doi: 10.1038/nrneph.2017.29. Epub 2017 Mar 13. Nat Rev Nephrol. 2017. PMID: 28287111 Free PMC article.
References
-
- Wei C, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

