Enhancing radiosensitization in EphB4 receptor-expressing Head and Neck Squamous Cell Carcinomas
- PMID: 27941840
- PMCID: PMC5150255
- DOI: 10.1038/srep38792
Enhancing radiosensitization in EphB4 receptor-expressing Head and Neck Squamous Cell Carcinomas
Abstract
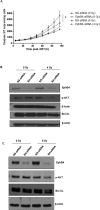
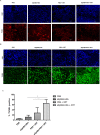
Members of the Eph family of receptor tyrosine kinases have been implicated in a wide array of human cancers. The EphB4 receptor is ubiquitously expressed in head and neck squamous cell carcinoma (HNSCC) and has been shown to impart tumorigenic and invasive characteristics to these cancers. In this study, we investigated whether EphB4 receptor targeting can enhance the radiosensitization of HNSCC. Our data show that EphB4 is expressed at high to moderate levels in HNSCC cell lines and patient-derived xenograft (PDX) tumors. We observed decreased survival fractions in HNSCC cells following EphB4 knockdown in clonogenic assays. An enhanced G2 cell cycle arrest with activation of DNA damage response pathway and increased apoptosis was evident in HNSCC cells following combined EphB4 downregulation and radiation compared to EphB4 knockdown and radiation alone. Data using HNSCC PDX models showed significant reduction in tumor volume and enhanced delay in tumor regrowth following sEphB4-HSA administration with radiation compared to single agent treatment. sEphB4-HSA is a protein known to block the interaction between the EphB4 receptor and its ephrin-B2 ligand. Overall, our findings emphasize the therapeutic relevance of EphB4 targeting as a radiosensitizer that can be exploited for the treatment of human head and neck carcinomas.
Figures




References
-
- Ang K. K. et al.. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 32, 2940–2950, doi: 10.1200/JCO.2013.53.5633 (2014). - DOI - PMC - PubMed
-
- Nguyen-Tan P. F. et al.. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol 32, 3858–3866, doi: 10.1200/JCO.2014.55.3925 (2014). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

