Self-preservation and structural transition of gas hydrates during dissociation below the ice point: an in situ study using Raman spectroscopy
- PMID: 27941857
- PMCID: PMC5150642
- DOI: 10.1038/srep38855
Self-preservation and structural transition of gas hydrates during dissociation below the ice point: an in situ study using Raman spectroscopy
Abstract
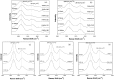
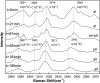
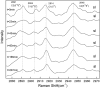
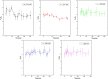
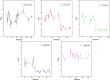
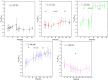
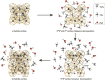
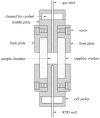
The hydrate structure type and dissociation behavior for pure methane and methane-ethane hydrates at temperatures below the ice point and atmospheric pressure were investigated using in situ Raman spectroscopic analysis. The self-preservation effect of sI methane hydrate is significant at lower temperatures (268.15 to 270.15 K), as determined by the stable C-H region Raman peaks and AL/AS value (Ratio of total peak area corresponding to occupancies of guest molecules in large cavities to small cavities) being around 3.0. However, it was reduced at higher temperatures (271.15 K and 272.15 K), as shown from the dramatic change in Raman spectra and fluctuations in AL/AS values. The self-preservation effect for methane-ethane double hydrate is observed at temperatures lower than 271.15 K. The structure transition from sI to sII occurred during the methane-ethane hydrate decomposition process, which was clearly identified by the shift in peak positions and the change in relative peak intensities at temperatures from 269.15 K to 271.15 K. Further investigation shows that the selectivity for self-preservation of methane over ethane leads to the structure transition; this kind of selectivity increases with decreasing temperature. This work provides new insight into the kinetic behavior of hydrate dissociation below the ice point.
Figures




Similar articles
-
Structural changes and preferential cage occupancy of ethane hydrate and methane-ethane mixed gas hydrate under very high pressure.J Chem Phys. 2008 Dec 14;129(22):224503. doi: 10.1063/1.3036006. J Chem Phys. 2008. PMID: 19071924
-
Kinetics of methane-ethane gas replacement in clathrate-hydrates studied by time-resolved neutron diffraction and Raman spectroscopy.J Phys Chem A. 2010 Jan 14;114(1):247-55. doi: 10.1021/jp908016j. J Phys Chem A. 2010. PMID: 19863115
-
Kinetic studies of methane-ethane mixed gas hydrates by neutron diffraction and Raman spectroscopy.J Phys Chem B. 2009 Apr 16;113(15):5172-80. doi: 10.1021/jp810248s. J Phys Chem B. 2009. PMID: 19354304
-
Direct observation of pressure-induced amorphization of methane/ethane hydrates using Raman and infrared spectroscopy.Phys Chem Chem Phys. 2023 Aug 23;25(33):22161-22170. doi: 10.1039/d3cp03096b. Phys Chem Chem Phys. 2023. PMID: 37564022
-
Dissociation enthalpies of synthesized multicomponent gas hydrates with respect to the guest composition and cage occupancy.J Phys Chem B. 2007 Aug 16;111(32):9539-45. doi: 10.1021/jp0712755. Epub 2007 Jul 20. J Phys Chem B. 2007. PMID: 17658742
Cited by
-
Synthesis of Methane Hydrate from Ice Powder Accelerated by Doping Ethanol into Methane Gas.Sci Rep. 2019 Aug 26;9(1):12345. doi: 10.1038/s41598-019-48832-8. Sci Rep. 2019. PMID: 31451712 Free PMC article.
-
Microscopic measurements on the decomposition behaviour of methane hydrates formed in natural sands.RSC Adv. 2019 May 13;9(26):14727-14735. doi: 10.1039/c9ra01611b. eCollection 2019 May 9. RSC Adv. 2019. PMID: 35516298 Free PMC article.
References
-
- Sloan E. D. & Koh C. A. Clathrate Hydrates of Natural Gases (third ed) Ch. 1, Overview and historical perspective, 1–5 & Ch. 2, Molecular structures and similarities to ice, 53–72 (CRC Press–Taylor & Francis, Boca Raton, FL, 2007).
-
- Sloan E. D. Fundamental principles and applications of natural gas hydrates. Nature 426, 353–359 (2003). - PubMed
-
- Jamaluddin A. K. M., Kalogerakis N. & Bishnoi P. R. Hydrate plugging problems in undersea natural gas pipelines under shutdown conditions. J. Petrol. Sci. Eng. 5, 323–335 (1991).
-
- Mahmood F. G., Hamid R. R. & Mahdi B. Investigation of hydrate formation in natural gas flow through underground transmission pipeline. J. Nat. Gas Sci. Eng. 15, 27–37 (2013).
-
- Peng B. Z. et al.. Flow characteristics and morphology of hydrate slurry formed from (natural gas + diesel oil/condensate oil + water) system containing anti-agglomerant. Chem. Eng. Sci. 84, 333–344 (2012).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

