Lactate signalling regulates fungal β-glucan masking and immune evasion
- PMID: 27941860
- PMCID: PMC5704895
- DOI: 10.1038/nmicrobiol.2016.238
Lactate signalling regulates fungal β-glucan masking and immune evasion
Abstract
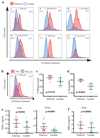
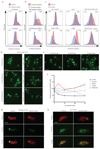
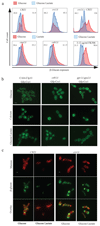
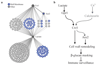
As they proliferate, fungi expose antigens at their cell surface that are potent stimulators of the innate immune response, and yet the commensal fungus Candida albicans is able to colonize immuno competent individuals. We show that C. albicans may evade immune detection by presenting a moving immunological target. We report that the exposure of β-glucan, a key pathogen-associated molecular pattern (PAMP) located at the cell surface of C. albicans and other pathogenic Candida species, is modulated in response to changes in the carbon source. Exposure to lactate induces β-glucan masking in C. albicans via a signalling pathway that has recruited an evolutionarily conserved receptor (Gpr1) and transcriptional factor (Crz1) from other well-characterized pathways. In response to lactate, these regulators control the expression of cell-wall-related genes that contribute to β-glucan masking. This represents the first description of active PAMP masking by a Candida species, a process that reduces the visibility of the fungus to the immune system.
Figures

Comment in
-
Immune evasion: Face changing in the fungal opera.Nat Microbiol. 2017 Jan 25;2:16266. doi: 10.1038/nmicrobiol.2016.266. Nat Microbiol. 2017. PMID: 28120932 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

