Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics
- PMID: 27941956
- PMCID: PMC5150989
- DOI: 10.1038/srep38915
Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics
Abstract
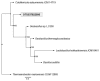
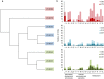
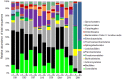
Composting is a promising source of new organisms and thermostable enzymes that may be helpful in environmental management and industrial processes. Here we present results of metagenomic- and metatranscriptomic-based analyses of a large composting operation in the São Paulo Zoo Park. This composting exhibits a sustained thermophilic profile (50 °C to 75 °C), which seems to preclude fungal activity. The main novelty of our study is the combination of time-series sampling with shotgun DNA, 16S rRNA gene amplicon, and metatranscriptome high-throughput sequencing, enabling an unprecedented detailed view of microbial community structure, dynamics, and function in this ecosystem. The time-series data showed that the turning procedure has a strong impact on the compost microbiota, restoring to a certain extent the population profile seen at the beginning of the process; and that lignocellulosic biomass deconstruction occurs synergistically and sequentially, with hemicellulose being degraded preferentially to cellulose and lignin. Moreover, our sequencing data allowed near-complete genome reconstruction of five bacterial species previously found in biomass-degrading environments and of a novel biodegrading bacterial species, likely a new genus in the order Bacillales. The data and analyses provided are a rich source for additional investigations of thermophilic composting microbiology.
Figures




References
-
- Ryckeboer J. et al. A survey of bacteria and fungi occurring during composting and self-heating processes. Annals of Microbiology 53, 349–410 (2003).
-
- Kumar S. Composting of municipal solid waste. Crit Rev Biotechnol 31, 112–136 (2011). - PubMed
-
- Alfreider A., Peters S., Tebbe C. C., Rangger A. & Insam H. Microbial community dynamics during composting of organic matter as determined by 16S ribosomal DNA analysis. Compost Sci Util 10, 303–312 (2002).
-
- Federici E. et al. Two-phase olive mill waste composting: Community dynamics and functional role of the resident microbiota. Bioresource Technology 102, 10965–10972 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

