MTOR-Driven Metabolic Reprogramming Regulates Legionella pneumophila Intracellular Niche Homeostasis
- PMID: 27942021
- PMCID: PMC5179073
- DOI: 10.1371/journal.ppat.1006088
MTOR-Driven Metabolic Reprogramming Regulates Legionella pneumophila Intracellular Niche Homeostasis
Abstract
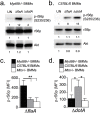
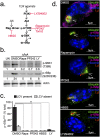
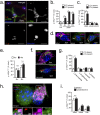
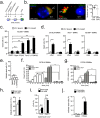
Vacuolar bacterial pathogens are sheltered within unique membrane-bound organelles that expand over time to support bacterial replication. These compartments sequester bacterial molecules away from host cytosolic immunosurveillance pathways that induce antimicrobial responses. The mechanisms by which the human pulmonary pathogen Legionella pneumophila maintains niche homeostasis are poorly understood. We uncovered that the Legionella-containing vacuole (LCV) required a sustained supply of host lipids during expansion. Lipids shortage resulted in LCV rupture and initiation of a host cell death response, whereas excess of host lipids increased LCVs size and housing capacity. We found that lipids uptake from serum and de novo lipogenesis are distinct redundant supply mechanisms for membrane biogenesis in Legionella-infected macrophages. During infection, the metabolic checkpoint kinase Mechanistic Target of Rapamycin (MTOR) controlled lipogenesis through the Serum Response Element Binding Protein 1 and 2 (SREBP1/2) transcription factors. In Legionella-infected macrophages a host-driven response that required the Toll-like receptors (TLRs) adaptor protein Myeloid differentiation primary response gene 88 (Myd88) dampened MTOR signaling which in turn destabilized LCVs under serum starvation. Inactivation of the host MTOR-suppression pathway revealed that L. pneumophila sustained MTOR signaling throughout its intracellular infection cycle by a process that required the upstream regulator Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and one or more Dot/Icm effector proteins. Legionella-sustained MTOR signaling facilitated LCV expansion and inhibition of the PI3K-MTOR-SREPB1/2 axis through pharmacological or genetic interference or by activation of the host MTOR-suppression response destabilized expanding LCVs, which in turn triggered cell death of infected macrophages. Our work identified a host metabolic requirement for LCV homeostasis and demonstrated that L. pneumophila has evolved to manipulate MTOR-dependent lipogenesis for optimal intracellular replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
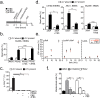
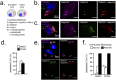
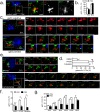

Comment in
-
The tug-of-war over MTOR in Legionella infections.Microb Cell. 2017 Jan 30;4(2):67-68. doi: 10.15698/mic2017.02.559. Microb Cell. 2017. PMID: 28357391 Free PMC article.
Similar articles
-
The Polar Legionella Icm/Dot T4SS Establishes Distinct Contact Sites with the Pathogen Vacuole Membrane.mBio. 2021 Oct 26;12(5):e0218021. doi: 10.1128/mBio.02180-21. Epub 2021 Oct 12. mBio. 2021. PMID: 34634944 Free PMC article.
-
Legionella-Containing Vacuoles Capture PtdIns(4)P-Rich Vesicles Derived from the Golgi Apparatus.mBio. 2018 Dec 11;9(6):e02420-18. doi: 10.1128/mBio.02420-18. mBio. 2018. PMID: 30538188 Free PMC article.
-
Comparative Proteomics of Purified Pathogen Vacuoles Correlates Intracellular Replication of Legionella pneumophila with the Small GTPase Ras-related protein 1 (Rap1).Mol Cell Proteomics. 2017 Apr;16(4):622-641. doi: 10.1074/mcp.M116.063453. Epub 2017 Feb 9. Mol Cell Proteomics. 2017. PMID: 28183814 Free PMC article.
-
Phosphoinositides and the Fate of Legionella in Phagocytes.Front Immunol. 2020 Jan 30;11:25. doi: 10.3389/fimmu.2020.00025. eCollection 2020. Front Immunol. 2020. PMID: 32117224 Free PMC article. Review.
-
Acanthamoeba and Dictyostelium as Cellular Models for Legionella Infection.Front Cell Infect Microbiol. 2018 Mar 2;8:61. doi: 10.3389/fcimb.2018.00061. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29552544 Free PMC article. Review.
Cited by
-
The Legionella effector WipB is a translocated Ser/Thr phosphatase that targets the host lysosomal nutrient sensing machinery.Sci Rep. 2017 Aug 25;7(1):9450. doi: 10.1038/s41598-017-10249-6. Sci Rep. 2017. PMID: 28842705 Free PMC article.
-
Regulation and repurposing of nutrient sensing and autophagy in innate immunity.Autophagy. 2021 Jul;17(7):1571-1591. doi: 10.1080/15548627.2020.1783119. Epub 2020 Jul 5. Autophagy. 2021. PMID: 32627660 Free PMC article. Review.
-
Legionella pneumophila, a Rosetta stone to understanding bacterial pathogenesis.J Bacteriol. 2024 Dec 19;206(12):e0032424. doi: 10.1128/jb.00324-24. Epub 2024 Dec 5. J Bacteriol. 2024. PMID: 39636264 Free PMC article. Review.
-
Glucosylation by the Legionella Effector SetA Promotes the Nuclear Localization of the Transcription Factor TFEB.iScience. 2020 Jul 24;23(7):101300. doi: 10.1016/j.isci.2020.101300. Epub 2020 Jun 20. iScience. 2020. PMID: 32622269 Free PMC article.
-
Iron-depleting nutritional immunity controls extracellular bacterial replication in Legionella pneumophila infections.Nat Commun. 2024 Sep 8;15(1):7848. doi: 10.1038/s41467-024-52184-x. Nat Commun. 2024. PMID: 39245746 Free PMC article.
References
-
- Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci. 2001;114: 4637–4650. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

