History and progression of Fat cadherins in health and disease
- PMID: 27942226
- PMCID: PMC5138043
- DOI: 10.2147/OTT.S111176
History and progression of Fat cadherins in health and disease
Abstract
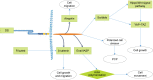
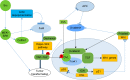
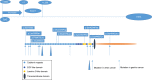
Intercellular adhesions are vital hubs for signaling pathways during multicellular development and animal morphogenesis. In eukaryotes, under aberrant intracellular conditions, cadherins are abnormally regulated, which can result in cellular pathologies such as carcinoma, kidney disease, and autoimmune diseases. As a member of the Ca2+-dependent adhesion super-family, Fat proteins were first described in the 1920s as an inheritable lethal mutant phenotype in Drosophila, consisting of four member proteins, FAT1, FAT2, FAT3, and FAT4, all of which are highly conserved in structure. Functionally, FAT1 was found to regulate cell migration and growth control through specific protein-protein interactions of its cytoplasmic tail. FAT2 and FAT3 are relatively less studied and are thought to participate in the development of human cancer through a pathway similar to that of the Ena/VASP proteins. In contrast, FAT4 has been widely studied in the context of biological functions and tumor mechanisms and has been shown to regulate the planar cell polarity pathway, the Hippo signaling pathway, the canonical Wnt signaling cascade, and the expression of YAP1. Overall, Fat cadherins may be useful as emerging disease biomarkers and as novel therapeutic targets.
Keywords: CpG island; FAT1; FAT2; FAT3; FAT4; Fat cadherins; Hippo pathway; WNT signaling.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Sadeqzadeh E, de Bock CE, Thorne RF. Sleeping giants: emerging roles for the fat cadherins in health and disease. Med Res Rev. 2014;34(1):190–221. - PubMed
-
- Tanoue T, Takeichi M. New insights into fat cadherins. J Cell Sci. 2005;118(pt 11):2347–2353. - PubMed
-
- Alders M, Al-Gazali L, Cordeiro I, et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum Genet. 2014;133(9):1161–1167. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

