ER Stress-induced Inflammasome Activation Contributes to Hepatic Inflammation and Steatosis
- PMID: 27942420
- PMCID: PMC5146989
- DOI: 10.4172/2155-9899.1000457
ER Stress-induced Inflammasome Activation Contributes to Hepatic Inflammation and Steatosis
Abstract
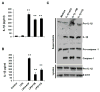
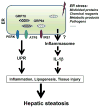
Endoplasmic reticulum (ER) stress functions as a protein folding and quality control mechanism to maintain cell homeostasis. Emerging evidence indicates that ER stress is also involved in metabolic and inflammatory diseases. However, the link between ER stress and inflammation remains not well characterized. In this study, we have demonstrated that ER stress-induced inflammasome activation plays a critical role in the pathogenesis of hepatic steatosis. By utilizing genetic and pharmacological agent-induced hepatic steatosis animal models, we found that hepatic steatosis was associated with inflammasome activation and ER stress. Our results show that caspase-1 ablation alleviated liver inflammation and injury. Liver tissues from caspase-1 KO mice had significantly reduced production of IL-1β under ER stress conditions. We also found that ER stress promoted inflammasome activation and IL-1β processing in both hepatocytes and Kupffer cells/macrophages. Moreover, lack of caspase-1 ameliorated cell death or pyropoptosis of hepatocytes induced by ER stress. Taken together, our findings suggest that ER stress-induced inflammasome activation and IL-1β production generate a positive feedback loop to amplify inflammatory response, eventually leading to liver steatosis and injury.
Keywords: Endoplasmic reticulum; Inflammasome; Liver; Steatosis.
Figures
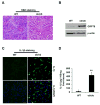
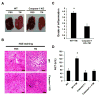


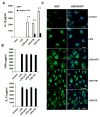
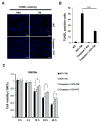
References
-
- Kaufman RJ. Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci. 2004;29:152–158. - PubMed
-
- Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. - PubMed
-
- Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
