Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease
- PMID: 27942582
- PMCID: PMC5135274
- DOI: 10.1172/jci.insight.87446
Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease
Abstract
Pentraxin-2 (PTX-2), also known as serum amyloid P component (SAP/APCS), is a constitutive, antiinflammatory, innate immune plasma protein whose circulating level is decreased in chronic human fibrotic diseases. Here we show that recombinant human PTX-2 (rhPTX-2) retards progression of chronic kidney disease in Col4a3 mutant mice with Alport syndrome, reducing blood markers of kidney failure, enhancing lifespan by 20%, and improving histological signs of disease. Exogenously delivered rhPTX-2 was detected in macrophages but also in tubular epithelial cells, where it counteracted macrophage activation and was cytoprotective for the epithelium. Computational analysis of genes regulated by rhPTX-2 identified the transcriptional regulator c-Jun along with its activator protein-1 (AP-1) binding partners as a central target for the function of rhPTX-2. Accordingly, PTX-2 attenuates c-Jun and AP-1 activity, and reduces expression of AP-1-dependent inflammatory genes in both monocytes and epithelium. Our studies therefore identify rhPTX-2 as a potential therapy for chronic fibrotic disease of the kidney and an important inhibitor of pathological c-Jun signaling in this setting.
Conflict of interest statement
R.M. Jack is the CSO, COO, and President of Promedior Inc. M.L. Lupher Jr. is a former member of Promedior Inc. J.S. Duffield holds stock options in Promedior Inc.
Figures
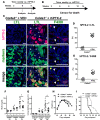
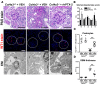
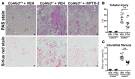

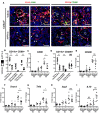


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

