Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes
- PMID: 27942583
- PMCID: PMC5135280
- DOI: 10.1172/jci.insight.88242
Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes
Abstract
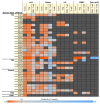
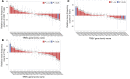
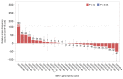
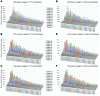
The adaptive immune repertoire plays a critical role in type 1 diabetes (T1D) pathogenesis. However, efforts to characterize B cell and T cell receptor (TCR) profiles in T1D subjects have been largely limited to peripheral blood sampling and restricted to known antigens. To address this, we collected pancreatic draining lymph nodes (pLN), "irrelevant" nonpancreatic draining lymph nodes, peripheral blood mononuclear cells (PBMC), and splenocytes from T1D subjects (n = 18) and control donors (n = 9) as well as pancreatic islets from 1 T1D patient; from these tissues, we collected purified CD4+ conventional T cells (Tconv), CD4+ Treg, CD8+ T cells, and B cells. By conducting high-throughput immunosequencing of the TCR β chain (TRB) and B cell receptor (BCR) immunoglobulin heavy chain (IGH) on these samples, we sought to analyze the molecular signature of the lymphocyte populations within these tissues and of T1D. Ultimately, we observed a highly tissue-restricted CD4+ repertoire, while up to 24% of CD8+ clones were shared among tissues. We surveyed our data set for previously described proinsulin- and glutamic acid decarboxylase 65-reactive (GAD65-reactive) receptors, and interestingly, we observed a TRB with homology to a known GAD65-reactive TCR (clone GAD4.13) present in 7 T1D donors (38.9%), representing >25% of all productive TRB within Tconv isolated from the pLN of 1 T1D subject. These data demonstrate diverse receptor signatures at the nucleotide level and enriched autoreactive clones at the amino acid level, supporting the utility of coupling immunosequencing data with knowledge of characterized autoreactive receptors.
Conflict of interest statement
M. Vignali, R.O. Emerson, and E. Yusko are employees of and have equity ownership in Adaptive Biotechnologies. H.S. Robins is an employee, has equity ownership, patents, and royalties with Adaptive Biotechnologies.
Figures


References
-
- Lampeter EF, McCann SR, Kolb H. Transfer of diabetes type 1 by bone-marrow transplantation. Lancet. 1998;351(9102):568–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

