Tumor-infiltrating lymphocytes are dynamically desensitized to antigen but are maintained by homeostatic cytokine
- PMID: 27942588
- PMCID: PMC5135268
- DOI: 10.1172/jci.insight.89289
Tumor-infiltrating lymphocytes are dynamically desensitized to antigen but are maintained by homeostatic cytokine
Abstract
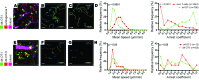
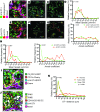
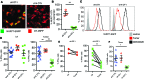
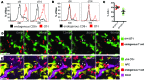
T cells that enter tumors are largely tolerized, but how that process is choreographed and how the ensuing "dysfunctional" tumor-infiltrating lymphocytes (TILs) are maintained are poorly understood and are difficult to assess in spontaneous disease. We exploited an autochthonous model of breast cancer for high-resolution imaging of the early and later stages of tumor residence to understand the relationships between cellular behaviors and cellular phenotypes. "Dysfunctional" differentiation began within the first days of tumor residence with an initial phase in which T cells arrest, largely on tumor-associated macrophages. Within 10 days, cellular motility increased and resembled a random walk, suggesting a relative absence of TCR signaling. We then studied the concurrent and apparently contradictory phenomenon that many of these cells express molecular markers of activation and were visualized undergoing active cell division. We found that whereas proliferation did not require ongoing TCR/ZAP70 signaling, instead this is driven in part by intratumoral IL-15 cytokine. Thus, TILs undergo sequential reprogramming by the tumor microenvironment and are actively retained, even while being antigen insensitive. We conclude that this program effectively fills the niche with ineffective yet cytokine-dependent TILs, and we propose that these might compete with new clones, when they arise.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

