Population Pharmacokinetics and Exploratory Pharmacodynamics of Lorazepam in Pediatric Status Epilepticus
- PMID: 27943220
- PMCID: PMC5466505
- DOI: 10.1007/s40262-016-0486-0
Population Pharmacokinetics and Exploratory Pharmacodynamics of Lorazepam in Pediatric Status Epilepticus
Abstract
Background: Lorazepam is one of the preferred agents used for intravenous treatment of status epilepticus (SE). We combined data from two pediatric clinical trials to characterize the population pharmacokinetics of intravenous lorazepam in infants and children aged 3 months to 17 years with active SE or a history of SE.
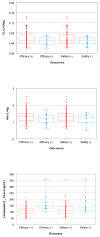
Methods: We developed a population pharmacokinetic model for lorazepam using the NONMEM software. We then assessed exploratory exposure-response relationships using the overall efficacy and safety study endpoints, and performed dosing simulations.
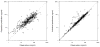
Results: A total of 145 patients contributed 439 pharmacokinetic samples. The median (range) age and dose were 5.4 years (0.3-17.8) and 0.10 mg/kg (0.02-0.18), respectively. A two-compartment pharmacokinetic model with allometric scaling described the data well. In addition to total body weight (WT), younger age was associated with slightly higher weight-normalized clearance (CL). The following relationships characterized the typical values for the central compartment volume (V1), CL, peripheral compartment volume (V2), and intercompartmental CL (Q), using individual subject WT (kg) and age (years): V1 (L) = 0.879*WT; CL (L/h) = 0.115*(Age/4.7)0.133*WT0.75; V2 (L) = 0.542*V1; Q (L/h) = 1.45*WT0.75. No pharmacokinetic parameters were associated with clinical outcomes. Simulations suggest uniform pediatric dosing (0.1 mg/kg, to a maximum of 4 mg) can be used to achieve concentrations of 50-100 ng/mL in children with SE, which have been previously associated with effective seizure control.
Conclusions: The population pharmacokinetics of lorazepam were successfully described using a sparse sampling approach and a two-compartment model in pediatric patients with active SE.
Conflict of interest statement
Figures


References
-
- Dham BS, Hunter K, Rincon F. The epidemiology of status epilepticus in the United States. Neurocrit Care. 2014;20:476–83. - PubMed
-
- Chin RFM, Neville BGR, Peckham C, Bedford H, Wade A, Scott RC. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368:222–9. - PubMed
-
- Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015;14:615–24. - PubMed
-
- Singh R, Gaillard W. Status epilepticus in children. Curr Neurol Neurosci Rep. 2009;9:137–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

