FV-100 versus valacyclovir for the prevention of post-herpetic neuralgia and the treatment of acute herpes zoster-associated pain: A randomized-controlled trial
- PMID: 27943311
- PMCID: PMC6139434
- DOI: 10.1002/jmv.24750
FV-100 versus valacyclovir for the prevention of post-herpetic neuralgia and the treatment of acute herpes zoster-associated pain: A randomized-controlled trial
Abstract
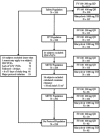
This prospective, parallel-group, randomized, double-blind, multicenter study compared the efficacy and safety of FV-100 with valacyclovir for reducing pain associated with acute herpes zoster (HZ). Patients, ≥50 years of age, diagnosed with HZ within 72 h of lesion appearance who had HZ-associated pain, were randomized 1:1:1 to a 7-day course of either FV-100 200 mg QD (n = 117), FV-100 400 mg QD (n = 116), or valacyclovir 1000 mg TID (n =117). Efficacy was evaluated on the basis of the burden of illness (BOI; Zoster Brief Pain Inventory scores); incidence and duration of clinically significant pain (CSP); pain scores; incidence and severity of post-herpetic neuralgia (PHN); and times to full lesion crusting and to lesion healing. Safety was evaluated on the basis of adverse event (AE)/SAE profiles, changes in laboratory and vital signs values, and results of electrocardiograms. The burden of illness scores for pain through 30 days were 114.5, 110.3, and 118.0 for FV-100 200 mg, FV-100 400 mg, and valacyclovir 3000 mg, respectively. The incidences of PHN at 90 days for FV-100 200 mg, FV-100 400 mg, and valacyclovir 3000 mg were 17.8%, 12.4%, and 20.2%, respectively. Adverse event and SAE profiles of the two FV-100 and the valacyclovir groups were similar and no untoward signals or trends were evident. These results demonstrate a potential for FV-100 as an antiviral for the treatment of shingles that could both reduce the pain burden of the acute episode and reduce the incidence of PHN compared with available treatments.
Trial registration: ClinicalTrials.gov NCT00900783.
Keywords: antiviral agents; herpes simplex virus; herpes virus; reinfection.
© 2016 Wiley Periodicals, Inc.
Figures
Similar articles
-
Open-label study of valacyclovir 1.5 g twice daily for the treatment of uncomplicated herpes zoster in immunocompetent patients 18 years of age or older.J Cutan Med Surg. 2007 May-Jun;11(3):89-98. doi: 10.2310/7750.2007.00016. J Cutan Med Surg. 2007. PMID: 17511925 Clinical Trial.
-
Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults.Antimicrob Agents Chemother. 1995 Jul;39(7):1546-53. doi: 10.1128/AAC.39.7.1546. Antimicrob Agents Chemother. 1995. PMID: 7492102 Free PMC article. Clinical Trial.
-
Efficacy of low dose gabapentin in acute herpes zoster for preventing postherpetic neuralgia: a prospective controlled study.Dermatol Ther. 2016 May;29(3):184-90. doi: 10.1111/dth.12331. Epub 2016 Jan 22. Dermatol Ther. 2016. PMID: 26799145 Clinical Trial.
-
Treatment of herpes zoster.Can Fam Physician. 2008 Mar;54(3):373-7. Can Fam Physician. 2008. PMID: 18337531 Free PMC article. Review.
-
Herpes zoster: diagnostic, therapeutic, and preventive approaches.Postgrad Med. 2013 Sep;125(5):78-91. doi: 10.3810/pgm.2013.09.2703. Postgrad Med. 2013. PMID: 24113666 Review.
Cited by
-
Current scenario and future applicability of antivirals against herpes zoster.Korean J Pain. 2023 Jan 1;36(1):4-10. doi: 10.3344/kjp.22391. Korean J Pain. 2023. PMID: 36573010 Free PMC article. Review.
-
Varicella Zoster Virus-Specific Hyperimmunoglobulin in the Adjuvant Treatment of Immunocompromised Herpes Zoster Patients: A Case Series.Dermatol Ther (Heidelb). 2023 Oct;13(10):2461-2471. doi: 10.1007/s13555-023-01019-6. Epub 2023 Sep 13. Dermatol Ther (Heidelb). 2023. PMID: 37704912 Free PMC article.
-
Herpes zoster after left nephroureterectomy for renal carcinoma: a case report.BMC Infect Dis. 2025 Jan 14;25(1):62. doi: 10.1186/s12879-025-10460-1. BMC Infect Dis. 2025. PMID: 39810115 Free PMC article.
-
Advances and Perspectives in the Management of Varicella-Zoster Virus Infections.Molecules. 2021 Feb 20;26(4):1132. doi: 10.3390/molecules26041132. Molecules. 2021. PMID: 33672709 Free PMC article. Review.
-
Recent Advances in Molecular Mechanisms of Nucleoside Antivirals.Curr Issues Mol Biol. 2023 Aug 17;45(8):6851-6879. doi: 10.3390/cimb45080433. Curr Issues Mol Biol. 2023. PMID: 37623252 Free PMC article. Review.
References
-
- Center for Disease Control and Prevention. What you need to know about the shingles vaccine. Available at: http://www.cdc.gov/vaccines/hcp/patient-ed/adults/downloads/fs-shingles.pdf Updated August 2014.
-
- Gnann JW, Jr , Whitley. Clinical practice. Herpes zoster. N Engl J Med. 2002; 347:340–346. - PubMed
-
- Schmader KE, Dworkin RH. Natural history and treatment of herpes zoster. J Pain. 2008; 9:3–9. - PubMed
-
- Arani RB, Soong SJ, Weiss HL, Wood MJ, Fiddian PA, Gnann JW, Whitley R. Phase specific analysis of herpes zoster associated pain data: A new statistical approach. Stat Med. 2001; 20:2429–2439. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

