Mechanism of Shiga Toxin Clustering on Membranes
- PMID: 27943675
- PMCID: PMC5348102
- DOI: 10.1021/acsnano.6b05706
Mechanism of Shiga Toxin Clustering on Membranes
Erratum in
-
Correction to Mechanism of Shiga Toxin Clustering on Membranes.ACS Nano. 2018 Feb 27;12(2):2079. doi: 10.1021/acsnano.8b00537. Epub 2018 Jan 25. ACS Nano. 2018. PMID: 29368915 Free PMC article. No abstract available.
Abstract
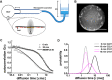
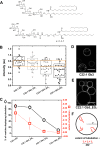
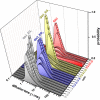
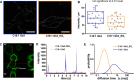
The bacterial Shiga toxin interacts with its cellular receptor, the glycosphingolipid globotriaosylceramide (Gb3 or CD77), as a first step to entering target cells. Previous studies have shown that toxin molecules cluster on the plasma membrane, despite the apparent lack of direct interactions between them. The precise mechanism by which this clustering occurs remains poorly defined. Here, we used vesicle and cell systems and computer simulations to show that line tension due to curvature, height, or compositional mismatch, and lipid or solvent depletion cannot drive the clustering of Shiga toxin molecules. By contrast, in coarse-grained computer simulations, a correlation was found between clustering and toxin nanoparticle-driven suppression of membrane fluctuations, and experimentally we observed that clustering required the toxin molecules to be tightly bound to the membrane surface. The most likely interpretation of these findings is that a membrane fluctuation-induced force generates an effective attraction between toxin molecules. Such force would be of similar strength to the electrostatic force at separations around 1 nm, remain strong at distances up to the size of toxin molecules (several nanometers), and persist even beyond. This force is predicted to operate between manufactured nanoparticles providing they are sufficiently rigid and tightly bound to the plasma membrane, thereby suggesting a route for the targeting of nanoparticles to cells for biomedical applications.
Keywords: Casimir force; clustering; endocytosis; fluctuation-induced force; glycosphingolipid; invagination; lectin; membrane.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

