Qualitative and quantitative results of interferon-γ release assays for monitoring the response to anti-tuberculosis treatment
- PMID: 27951621
- PMCID: PMC5339471
- DOI: 10.3904/kjim.2016.199
Qualitative and quantitative results of interferon-γ release assays for monitoring the response to anti-tuberculosis treatment
Abstract
Background/aims: The usefulness of interferon-γ release assays (IGRAs) in monitoring to responses to anti-tuberculosis (TB) treatment is controversial. We compared the results of two IGRAs before and after anti-TB treatment in same patients with active TB.
Methods: From a retrospective review, we selected patients with active TB who underwent repeated QuantiFERON-TB Gold (QFN-Gold, Cellestis Limited) and T-SPOT.TB (Oxford Immunotec) assays before and after anti-TB treatment with first-line drugs. Both tests were performed prior to the start of anti-TB treatment or within 1 week after the start of anti-TB treatment and after completion of treatment.
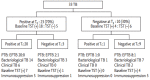
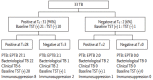
Results: A total of 33 active TB patients were included in the study. On the QFN-Gold test, at baseline, 23 cases (70%) were early secreted antigenic target 6-kDa protein 6 (ESAT-6) or culture filtrate protein 10 (CFP-10) positive. On the T-SPOT. TB test, at baseline, 31 cases (94%) were ESAT-6 or CFP-10 positive. Most of patients remained both test-positive after anti-TB treatment. Although changes in interferon-γ release responses over time were highly variable in both tests, there was a mean decline of 27 and 24 spot-forming counts for ESAT-6 and CFP-10, respectively on the T-SPOT.TB test (p < 0.05 for all).
Conclusions: Although limited by the small number of patients and a short-term follow-up, there was significant decline in the quantitative result of the T-SPOT. TB test with treatment. However, both commercial IGRAs may not provide evidence regarding the cure of disease in Korea, a country where the prevalence of TB is within the intermediate range.
Keywords: Interferon-gamma release tests; Therapeutics; Tuberculosis.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


References
-
- World Health Organization . Global Tuberculosis Report 2015. Geneva: World Health Organization; 2015.
-
- World Health Organization . Treatment of Tuberculosis: Guidelines for National Programmes. 3rd ed. Geneva: World Health Organization; 2003.
-
- Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection: United States, 2010. MMWR Recomm Rep. 2010;59:1–25. - PubMed
-
- Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004;364:2196–2203. - PubMed
-
- Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A, et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
