Depletion of SAG/RBX2 E3 ubiquitin ligase suppresses prostate tumorigenesis via inactivation of the PI3K/AKT/mTOR axis
- PMID: 27955654
- PMCID: PMC5153812
- DOI: 10.1186/s12943-016-0567-6
Depletion of SAG/RBX2 E3 ubiquitin ligase suppresses prostate tumorigenesis via inactivation of the PI3K/AKT/mTOR axis
Abstract
Background: SAG (Sensitive to Apoptosis Gene), also known as RBX2, ROC2 or RNF7, is a RING component of CRL (Cullin-RING ligase), required for its activity. Our recent study showed that SAG/RBX2 co-operated with Kras to promote lung tumorigenesis, but antagonized Kras to inhibit skin tumorigenesis, suggesting a tissue/context dependent function of Sag. However, it is totally unknown whether and how Sag would play in prostate tumorigenesis, triggered by Pten loss.
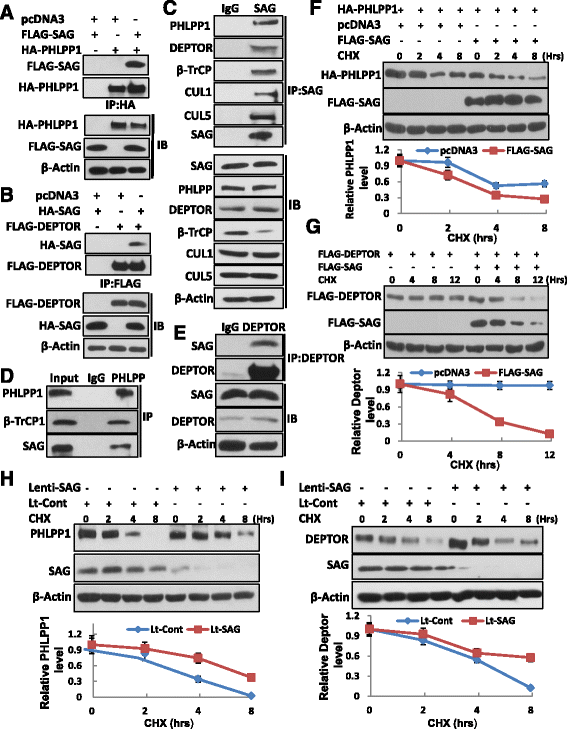
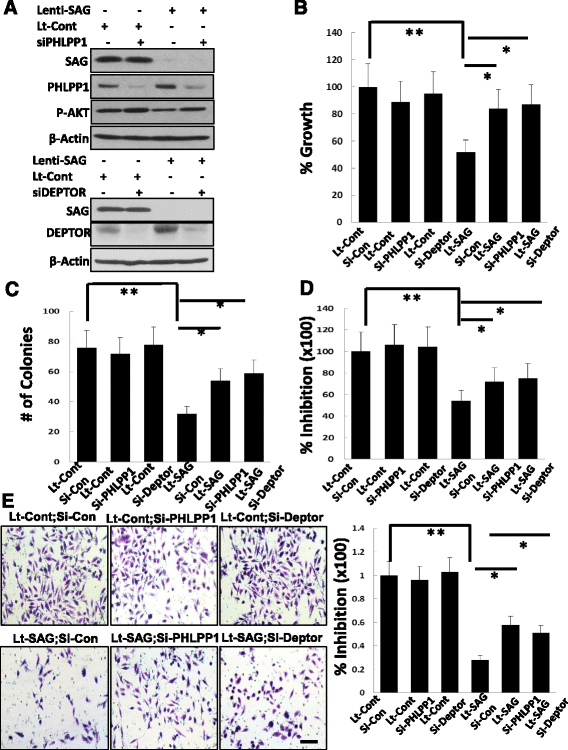
Methods: Sag and Pten double conditional knockout mice were generated and prostate specific deletion of Sag and Pten was achieved by PB4-Cre, and their effect on prostate tumorigenesis was evaluated by H&E staining. The methods of immunohistochemistry (IHC) staining and Western blotting were utilized to examine expression of various proteins in prostate cancer tissues or cell lines. The effect of SAG knockdown in proliferation, survival and migration was evaluated in two prostate cancer cell lines. The poly-ubiquitylation of PHLPP1 and DEPTOR was evaluated by both in vivo and in vitro ubiquitylation assays.
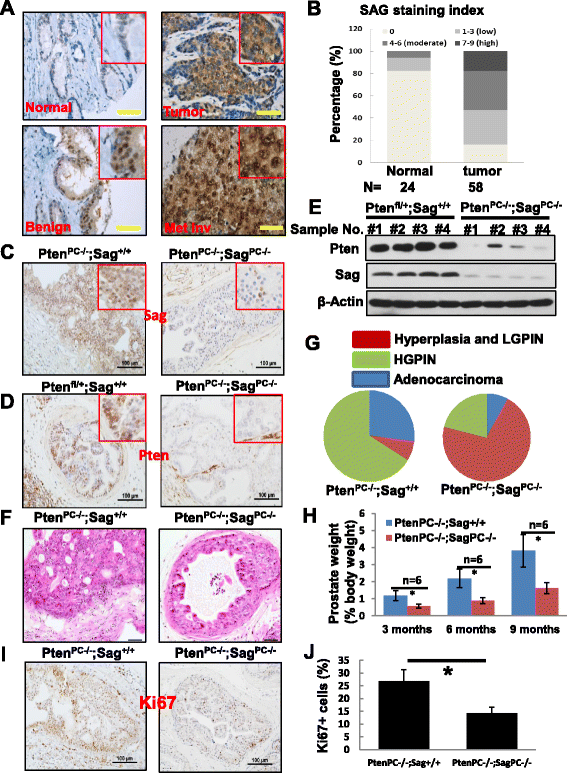
Results: SAG is overexpressed progressively from early-to-late stage of human prostate cancer with the highest expression seen in metastatic lesion. Sag deletion inhibits prostate tumorigenesis triggered by Pten loss in a mouse model as a result of suppressed proliferation. SAG knockdown in human prostate cancer cells inhibits a) proliferation in monolayer and soft agar, b) clonogenic survival, and c) migration. SAG is an E3 ligase that promotes ubiquitylation and degradation of PHLPP1 and DEPTOR, leading to activation of the PI3K/AKT/mTOR axis, whereas SAG knockdown caused their accumulation. Importantly, growth suppression triggered by SAG knockdown was partially rescued by simultaneous knockdown of PHLPP1 or DEPTOR, suggesting their causal role. Accumulation of Phlpp1 and Deptor with corresponding inactivation of Akt/mTOR was also detected in Sag-null prostate cancer tissues.
Conclusions: Sag is an oncogenic cooperator of Pten-loss for prostate tumorigenesis. Targeting SAG E3 ligase may, therefore, have therapeutic value for the treatment of prostate cancer associated with Pten loss.
Keywords: DEPTOR; PHLPP1; Prostate tumorigenesis; Pten; SAG KO; SAG-SCF E3; Ubiquitin ligase.
Figures



Similar articles
-
Inactivation of Sag/Rbx2/Roc2 e3 ubiquitin ligase triggers senescence and inhibits kras-induced immortalization.Neoplasia. 2015 Jan;17(1):114-23. doi: 10.1016/j.neo.2014.11.008. Neoplasia. 2015. PMID: 25622904 Free PMC article.
-
Transgenic expression of Sag/Rbx2 E3 causes early stage tumor promotion, late stage cytogenesis and acinar loss in the Kras-PDAC model.Neoplasia. 2020 Jun;22(6):242-252. doi: 10.1016/j.neo.2020.03.002. Epub 2020 Apr 24. Neoplasia. 2020. PMID: 32339950 Free PMC article.
-
Inactivation of SAG/RBX2 E3 ubiquitin ligase suppresses KrasG12D-driven lung tumorigenesis.J Clin Invest. 2014 Feb;124(2):835-46. doi: 10.1172/JCI70297. Epub 2014 Jan 16. J Clin Invest. 2014. PMID: 24430184 Free PMC article.
-
Functional characterization of SAG/RBX2/ROC2/RNF7, an antioxidant protein and an E3 ubiquitin ligase.Protein Cell. 2013 Feb;4(2):103-16. doi: 10.1007/s13238-012-2105-7. Epub 2012 Nov 8. Protein Cell. 2013. PMID: 23136067 Free PMC article. Review.
-
Targeting the mTOR-DEPTOR pathway by CRL E3 ubiquitin ligases: therapeutic application.Neoplasia. 2012 May;14(5):360-7. doi: 10.1593/neo.12532. Neoplasia. 2012. PMID: 22745582 Free PMC article. Review.
Cited by
-
The UBE2C/CDH1/DEPTOR axis is an oncogene and tumor suppressor cascade in lung cancer cells.J Clin Invest. 2023 Feb 15;133(4):e162434. doi: 10.1172/JCI162434. J Clin Invest. 2023. PMID: 36548081 Free PMC article.
-
SAG expression associates with COPB2-related signaling and a poorer prognosis in breast cancer.Aging (Albany NY). 2020 Jan 11;12(1):902-911. doi: 10.18632/aging.102663. Epub 2020 Jan 11. Aging (Albany NY). 2020. PMID: 31926110 Free PMC article.
-
LINC01088/miR-22/CDC6 Axis Regulates Prostate Cancer Progression by Activating the PI3K/AKT Pathway.Mediators Inflamm. 2023 Jul 19;2023:9207148. doi: 10.1155/2023/9207148. eCollection 2023. Mediators Inflamm. 2023. PMID: 37501932 Free PMC article.
-
Identification of a Ubiquitination-Related Gene Risk Model for Predicting Survival in Patients With Pancreatic Cancer.Front Genet. 2020 Dec 22;11:612196. doi: 10.3389/fgene.2020.612196. eCollection 2020. Front Genet. 2020. PMID: 33414811 Free PMC article.
-
The androgen receptor inhibits transcription of GPER1 by preventing Sp1 and Sp3 from binding to the promoters in prostate cancer cells.Oncotarget. 2022 Jan 7;13:46-60. doi: 10.18632/oncotarget.28169. eCollection 2022. Oncotarget. 2022. PMID: 35018219 Free PMC article.
References
-
- Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

