Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence
- PMID: 27955712
- PMCID: PMC5153817
- DOI: 10.1186/s12916-016-0733-0
Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence
Abstract
Background: Pregnancy triggers well-known alterations in maternal glucose and lipid balance but its overall effects on systemic metabolism remain incompletely understood.
Methods: Detailed molecular profiles (87 metabolic measures and 37 cytokines) were measured for up to 4260 women (24-49 years, 322 pregnant) from three population-based cohorts in Finland. Circulating molecular concentrations in pregnant women were compared to those in non-pregnant women. Metabolic profiles were also reassessed for 583 women 6 years later to uncover the longitudinal metabolic changes in response to change in the pregnancy status.
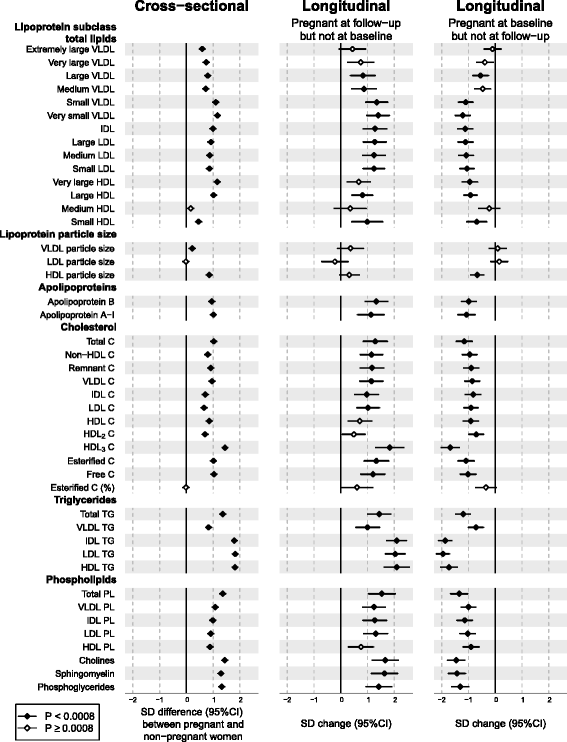
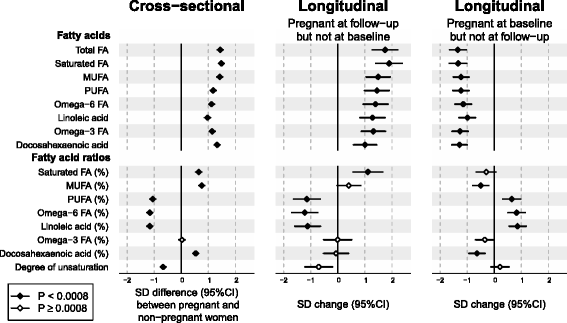
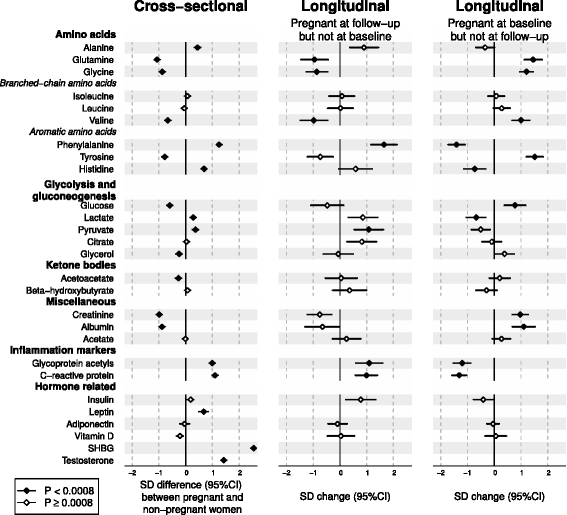
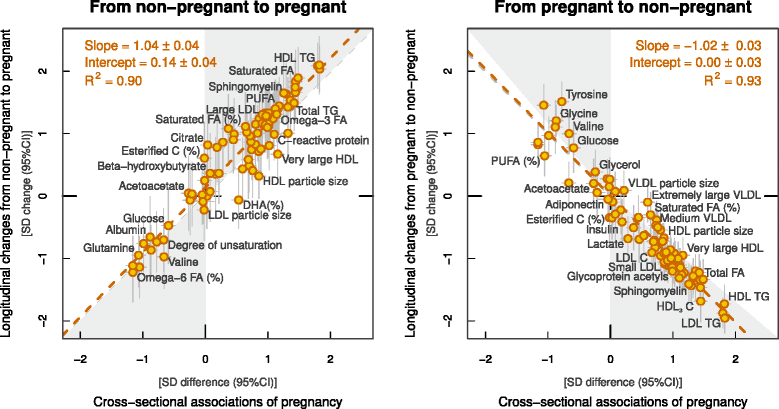
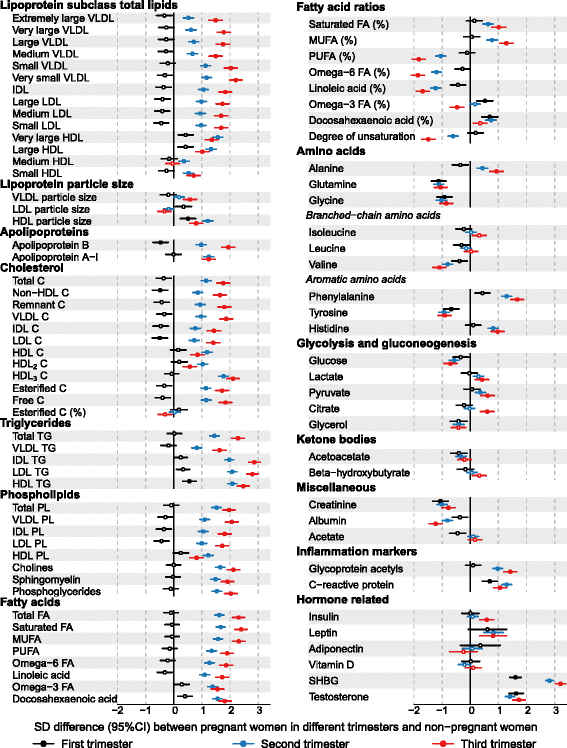
Results: Compared to non-pregnant women, all lipoprotein subclasses and lipids were markedly increased in pregnant women. The most pronounced differences were observed for the intermediate-density, low-density and high-density lipoprotein triglyceride concentrations. Large differences were also seen for many fatty acids and amino acids. Pregnant women also had higher concentrations of low-grade inflammatory marker glycoprotein acetyls, higher concentrations of interleukin-18 and lower concentrations of interleukin-12p70. The changes in metabolic concentrations for women who were not pregnant at baseline but pregnant 6 years later (or vice versa) matched (or were mirror-images of) the cross-sectional association pattern. Cross-sectional results were consistent across the three cohorts and similar longitudinal changes were seen for 653 women in 4-year and 497 women in 10-year follow-up. For multiple metabolic measures, the changes increased in magnitude across the three trimesters.
Conclusions: Pregnancy initiates substantial metabolic and inflammatory changes in the mothers. Comprehensive characterisation of normal pregnancy is important for gaining understanding of the key nutrients for fetal growth and development. These findings also provide a valuable molecular reference in relation to studies of adverse pregnancy outcomes.
Keywords: Amino acids; Cytokines; Fatty acids; Hormones; Inflammation; Lipoprotein lipids; Metabolic networks; Metabolomics; Postpartum; Pregnancy; Trimesters.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

