Salmonella Meningitis Associated with Monocyte Infiltration in Mice
- PMID: 27955815
- PMCID: PMC5225301
- DOI: 10.1016/j.ajpath.2016.09.002
Salmonella Meningitis Associated with Monocyte Infiltration in Mice
Abstract
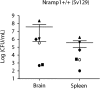
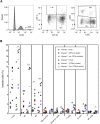
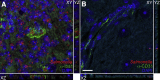
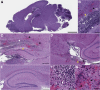
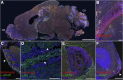
In the current study, we examined the ability of Salmonella enterica serovar Typhimurium to infect the central nervous system and cause meningitis following the natural route of infection in mice. C57BL/6J mice are extremely susceptible to systemic infection by Salmonella Typhimurium because of loss-of-function mutations in Nramp1 (SLC11A1), a phagosomal membrane protein that controls iron export from vacuoles and inhibits Salmonella growth in macrophages. Therefore, we assessed the ability of Salmonella to disseminate to the central nervous system (CNS) after oral infection in C57BL/6J mice expressing either wild-type (resistant) or mutant (susceptible) alleles of Nramp1. In both strains, oral infection resulted in focal meningitis and ventriculitis with recruitment of inflammatory monocytes to the CNS. In susceptible Nramp1-/- mice, there was a direct correlation between bacteremia and the number of bacteria in the brain, which was not observed in resistant Nramp1+/+ mice. A small percentage of Nramp1+/+ mice developed severe ataxia, which was associated with high bacterial loads in the CNS as well as clear histopathology of necrotizing vasculitis and hemorrhage in the brain. Thus, Nramp1 is not essential for Salmonella entry into the CNS or neuroinflammation, but may influence the mechanisms of CNS entry as well as the severity of meningitis.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

