Axial levodopa-induced dyskinesias and neuronal activity in the dorsal striatum
- PMID: 27956068
- PMCID: PMC5262537
- DOI: 10.1016/j.neuroscience.2016.11.046
Axial levodopa-induced dyskinesias and neuronal activity in the dorsal striatum
Abstract
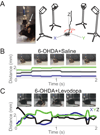
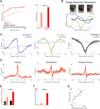
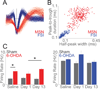
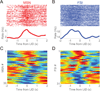
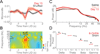
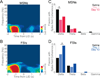
Levodopa-induced dyskinesias are abnormal involuntary movements that limit the effectiveness of treatments for Parkinson's disease. Although dyskinesias involve the striatum, it is unclear how striatal neurons are involved in dyskinetic movements. Here we record from striatal neurons in mice during levodopa-induced axial dyskinesias. We developed an automated 3-dimensional motion tracking system to capture the development of axial dyskinesias at ∼10ms resolution, and correlated these movements with neuronal activity of striatal medium spiny neurons and fast-spiking interneurons. The average firing rate of medium spiny neurons increased as axial dyskinesias developed, and both medium spiny neurons and fast-spiking interneurons were modulated around axial dyskinesias. We also found that delta field potential power increased in the striatum with dyskinesia, and that this increased delta power coupled with striatal neurons. Our findings provide insight into how striatal networks change as levodopa-induced dyskinesias develop, and suggest that increased medium spiny neuron firing, increased delta field potential power, and abnormal delta-coupling may be neurophysiological signatures of dyskinesias. These data could be helpful in understanding the role of the striatum in the pathogenesis of dyskinesias in Parkinson's disease.
Keywords: Parkinson’s disease; fast-spiking interneurons; levodopa-induced dyskinesia; medium spiny neurons; tracking.
Copyright © 2016 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord Off J Mov Disord Soc. 2001;16:448–458. - PubMed
-
- Aubert I, Guigoni C, Håkansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. - PubMed
-
- Barter JW, Li S, Lu D, Bartholomew RA, Rossi MA, Shoemaker CT, Salas-Meza D, Gaidis E, Yin HH. Beyond reward prediction errors: the role of dopamine in movement kinematics. [Accessed January 26, 2016];Front Integr Neurosci. 2015a 9 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4444742/ - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

