Effects of Oritavancin on Coagulation Tests in the Clinical Laboratory
- PMID: 27956417
- PMCID: PMC5278725
- DOI: 10.1128/AAC.01968-16
Effects of Oritavancin on Coagulation Tests in the Clinical Laboratory
Abstract
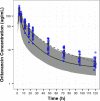
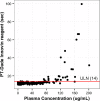
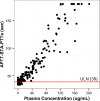
Previous studies have shown that some lipoglycopeptide and lipopeptide antimicrobial agents may cause falsely elevated values for some phospholipid-dependent coagulation tests. The effect of oritavancin, a lipoglycopeptide antibiotic, on coagulation test results was explored using pooled human plasma samples spiked with drug and in a clinical study after an infusion of a single 1,200-mg intravenous dose of oritavancin in normal healthy volunteers. Pooled plasma with oritavancin added ex vivo showed concentration-dependent prolongation of prothrombin time/international normalized ratio (PT/INR), activated partial thromboplastin time (aPTT), and dilute Russell viper venom time (DRVVT) test results. In contrast, oritavancin had no effect on the activated protein C resistance assay, chromogenic anti-factor Xa assay (anti-FXa), thrombin time, and an immunoassay for the laboratory diagnosis of heparin-induced thrombocytopenia. In participants that received a single dose of oritavancin, elevations in PT/INR result, aPTT, DRVVT, activated clotting time, and silica clotting time occurred, with the maximum times to resolution of test interference determined to be 12, 120, 72, 24, and 18 h, respectively. The anti-FXa assay was unaffected, whereas transient elevations in D dimer levels were observed in 30% of participants, with a maximum time to resolution of 72 h. Although oritavancin has no impact on the coagulation system in vivo, a single dose of oritavancin can produce falsely elevated values of some coagulation tests used to monitor hemostasis. The interference of oritavancin on affected tests is transient, and the test results revert to normal ranges within specified times after dosing.
Keywords: ABSSSI; antibiotic; coagulation; hemostasis; lipoglycopeptide; oritavancin.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O'Riordan W, SOLO I Investigators. 2014. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 370:2180–2190. doi:10.1056/NEJMoa1310422. - DOI - PubMed
-
- Corey GR, Good S, Jiang H, Moeck G, Wikler M, Green S, Manos P, Keech R, Singh R, Heller B, Bubnova N, O'Riordan W, SOLO II Investigators. 2015. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis 60:254–262. doi:10.1093/cid/ciu778. - DOI - PubMed
-
- . Orbactiv® (oritavancin) for injection, for intravenous use. Prescribing information, January 2016 ed. The Medicines Company, Parsippany, NJ.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

