Catalytic Mechanism of a Novel Glycoside Hydrolase Family 16 "Elongating" β-Transglycosylase
- PMID: 27956553
- PMCID: PMC5290943
- DOI: 10.1074/jbc.M116.762419
Catalytic Mechanism of a Novel Glycoside Hydrolase Family 16 "Elongating" β-Transglycosylase
Abstract
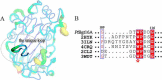
Carbohydrates are complex macromolecules in biological metabolism. Enzymatic synthesis of carbohydrates is recognized as a powerful tool to overcome the problems associated with large scale synthesis of carbohydrates. Novel enzymes with significant transglycosylation ability are still in great demand in glycobiology studies. Here we report a novel glycoside hydrolase family 16 "elongating" β-transglycosylase from Paecilomyces thermophila (PtBgt16A), which efficiently catalyzes the synthesis of higher polymeric oligosaccharides using β-1,3/1,4-oligosaccharides as donor/acceptor substrates. Further structural information reveals that PtBgt16A has a binding pocket around the -1 subsite. The catalytic mechanism of PtBgt16A is partly similar to an exo-glycoside hydrolase, which cleaves the substrate from the non-reducing end one by one. However, PtBgt16A releases the reducing end product and uses the remainder glucosyl as a transglycosylation donor. This catalytic mechanism has similarity with the catalytic mode of amylosucrase, which catalyzes the transglycosylation products gradually extend by one glucose unit. PtBgt16A thus has the potential to be a tool enzyme for the enzymatic synthesis of new β-oligosaccharides and glycoconjugates.
Keywords: enzyme catalysis; enzyme mechanism; enzyme structure; glycoside hydrolase; protein crystallization.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
Structural basis for transglycosylation in glycoside hydrolase family GH116 glycosynthases.Arch Biochem Biophys. 2021 Jul 30;706:108924. doi: 10.1016/j.abb.2021.108924. Epub 2021 May 18. Arch Biochem Biophys. 2021. PMID: 34019851
-
Structural and mutagenetic analyses of a 1,3-1,4-β-glucanase from Paecilomyces thermophila.Biochim Biophys Acta. 2014 Feb;1844(2):366-73. doi: 10.1016/j.bbapap.2013.11.005. Epub 2013 Nov 18. Biochim Biophys Acta. 2014. PMID: 24262091
-
Degradation and synthesis of β-glucans by a Magnaporthe oryzae endotransglucosylase, a member of the glycoside hydrolase 7 family.J Biol Chem. 2013 May 10;288(19):13821-30. doi: 10.1074/jbc.M112.448902. Epub 2013 Mar 25. J Biol Chem. 2013. PMID: 23530038 Free PMC article.
-
Enzymatic synthesis of oligosaccharides by two glycosyl hydrolases of Sulfolobus solfataricus.Extremophiles. 2001 Jun;5(3):145-52. doi: 10.1007/s007920100186. Extremophiles. 2001. PMID: 11453457 Review.
-
Synthesis of Human Milk Oligosaccharides: Protein Engineering Strategies for Improved Enzymatic Transglycosylation.Molecules. 2019 May 28;24(11):2033. doi: 10.3390/molecules24112033. Molecules. 2019. PMID: 31141914 Free PMC article. Review.
Cited by
-
A Novel Dimeric Exoglucanase (GH5_38): Biochemical and Structural Characterisation towards its Application in Alkyl Cellobioside Synthesis.Molecules. 2020 Feb 9;25(3):746. doi: 10.3390/molecules25030746. Molecules. 2020. PMID: 32050450 Free PMC article.
-
Molecular Basis for Substrate Recognition and Catalysis by a Marine Bacterial Laminarinase.Appl Environ Microbiol. 2020 Nov 10;86(23):e01796-20. doi: 10.1128/AEM.01796-20. Print 2020 Nov 10. Appl Environ Microbiol. 2020. PMID: 32917756 Free PMC article.
-
Biochemical Characterization of a Novel Endo-1,3-β-Glucanase from the Scallop Chlamys farreri.Mar Drugs. 2020 Sep 16;18(9):466. doi: 10.3390/md18090466. Mar Drugs. 2020. PMID: 32947865 Free PMC article.
-
A subfamily roadmap of the evolutionarily diverse glycoside hydrolase family 16 (GH16).J Biol Chem. 2019 Nov 1;294(44):15973-15986. doi: 10.1074/jbc.RA119.010619. Epub 2019 Sep 9. J Biol Chem. 2019. PMID: 31501245 Free PMC article.
-
Insights into the dual cleavage activity of the GH16 laminarinase enzyme class on β-1,3 and β-1,4 glycosidic bonds.J Biol Chem. 2021 Jan-Jun;296:100385. doi: 10.1016/j.jbc.2021.100385. Epub 2021 Feb 5. J Biol Chem. 2021. PMID: 33556371 Free PMC article.
References
-
- Cobucci-Ponzano B., Strazzulli A., Rossi M., and Moracci M. (2011) Glycosynthases in biocatalysis. Adv. Synth. Catal. 353, 2284–2300
-
- Bissaro B., Monsan P., Fauré R., and O'Donohue M. J. (2015) Glycosynthesis in a waterworld: new insight into the molecular basis of transglycosylation in retaining glycoside hydrolases. Biochem. J. 467, 17–35 - PubMed
-
- Cobucci-Ponzano B., and Moracci M. (2012) Glycosynthases as tools for the production of glycan analogs of natural products. Nat. Prod. Rep. 29, 697–709 - PubMed
-
- Spadiut O., Ibatullin F. M., Peart J., Gullfot F., Martinez-Fleites C., Ruda M., Xu C., Sundqvist G., Davies G. J., and Brumer H. (2011) Building custom polysaccharides in vitro with an efficient, broad-specificity xyloglucan glycosynthase and a fucosyltransferase. J. Am. Chem. Soc. 133, 10892–10900 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

