Modeling synovial sarcoma metastasis in the mouse: PI3'-lipid signaling and inflammation
- PMID: 27956588
- PMCID: PMC5154942
- DOI: 10.1084/jem.20160817
Modeling synovial sarcoma metastasis in the mouse: PI3'-lipid signaling and inflammation
Abstract
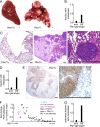
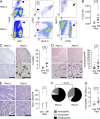
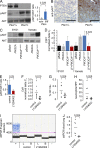
Solid tumor metastasis is a complex biology, impinged upon by a variety of dysregulated signaling pathways. PI3'-lipid signaling has been associated with metastasis and inflammation in many cancers, but the relationship between tumor cell-intrinsic PI3'-lipid signaling and inflammatory cell recruitment has remained enigmatic. Elevated PI3'-lipid signaling associates with progression of synovial sarcoma, a deadly soft tissue malignancy initiated by a t(X;18) chromosomal translocation that generates an SS18-SSX fusion oncoprotein. Here, we show in genetically engineered mouse models of locally induced expression of SS18-SSX1 or SS18-SSX2 that Pten silencing dramatically accelerated and enhanced sarcomagenesis without compromising synovial sarcoma characteristics. PTEN deficiency increased tumor angiogenesis, promoted inflammatory gene expression, and enabled highly penetrant spontaneous pulmonary metastasis. PTEN-deficient sarcomas revealed infiltrating myeloid-derived hematopoietic cells, particularly macrophages and neutrophils, recruited via PI3'-lipid-induced CSF1 expression in tumor cells. Moreover, in a large panel of human synovial sarcomas, enhanced PI3'-lipid signaling also correlated with increased inflammatory cell recruitment and CSF1R signal transduction in both macrophages and endothelial cells. Thus, both in the mouse model and in human synovial sarcomas, PI3'-lipid signaling drives CSF1 expression and associates with increased infiltration of the monocyte/macrophage lineage as well as neutrophils.
© 2016 Barrott et al.
Figures





References
-
- Abate-Shen C., Banach-Petrosky W.A., Sun X., Economides K.D., Desai N., Gregg J.P., Borowsky A.D., Cardiff R.D., and Shen M.M.. 2003. Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 63:3886–3890. - PubMed
-
- Bald T., Quast T., Landsberg J., Rogava M., Glodde N., Lopez-Ramos D., Kohlmeyer J., Riesenberg S., van den Boorn-Konijnenberg D., Hömig-Hölzel C., et al. 2014. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 507:109–113. 10.1038/nature13111 - DOI - PubMed
-
- Barrott J.J., Illum B.E., Jin H., Zhu J.F., Mosbruger T., Monument M.J., Smith-Fry K., Cable M.G., Wang Y., Grossmann A.H., et al. 2015. β-catenin stabilization enhances SS18-SSX2-driven synovial sarcomagenesis and blocks the mesenchymal to epithelial transition. Oncotarget. 6:22758–22766. 10.18632/oncotarget.4283 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

