Interrogating the Escherichia coli cell cycle by cell dimension perturbations
- PMID: 27956612
- PMCID: PMC5206551
- DOI: 10.1073/pnas.1617932114
Interrogating the Escherichia coli cell cycle by cell dimension perturbations
Abstract
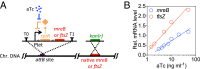
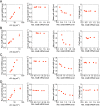
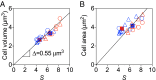
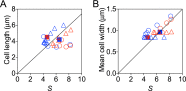
Bacteria tightly regulate and coordinate the various events in their cell cycles to duplicate themselves accurately and to control their cell sizes. Growth of Escherichia coli, in particular, follows a relation known as Schaechter's growth law. This law says that the average cell volume scales exponentially with growth rate, with a scaling exponent equal to the time from initiation of a round of DNA replication to the cell division at which the corresponding sister chromosomes segregate. Here, we sought to test the robustness of the growth law to systematic perturbations in cell dimensions achieved by varying the expression levels of mreB and ftsZ We found that decreasing the mreB level resulted in increased cell width, with little change in cell length, whereas decreasing the ftsZ level resulted in increased cell length. Furthermore, the time from replication termination to cell division increased with the perturbed dimension in both cases. Moreover, the growth law remained valid over a range of growth conditions and dimension perturbations. The growth law can be quantitatively interpreted as a consequence of a tight coupling of cell division to replication initiation. Thus, its robustness to perturbations in cell dimensions strongly supports models in which the timing of replication initiation governs that of cell division, and cell volume is the key phenomenological variable governing the timing of replication initiation. These conclusions are discussed in the context of our recently proposed "adder-per-origin" model, in which cells add a constant volume per origin between initiations and divide a constant time after initiation.
Keywords: DNA replication; E. coli; cell cycle; cell dimension; cell growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Amir A. Cell size regulation in bacteria. Phys Rev Lett. 2014;112(20):208102.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

