Functionalizing the γ-Position of α-Diazo-β-ketoesters
- PMID: 27956752
- PMCID: PMC5147747
- DOI: 10.1016/j.tetlet.2016.06.065
Functionalizing the γ-Position of α-Diazo-β-ketoesters
Abstract
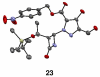
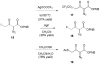
Although α-diazo-β-ketoesters are synthetically versatile intermediates, methodology for introducing this functionality into complex molecules is still limited, most frequently involving a carboxylic acid precursor, which is then activated and transformed into a β-ketoester, with the diazo group being subsequently added with a diazo transfer reagent. While introducing this highly functional moiety in a convergent one step process would be ideal, such an objective is limited by the relatively few studies which address functionalization of the α-diazo-β-ketoester at the γ-position. In the present investigation, we evaluate strategies, both new and established, for functionalizing α-diazo-β-ketoesters, particularly with regard to generating compounds prospectively useful in the synthesis of C1-substituted carbapenems. We report the first δ-aldehydo-α-diazo-β-ketoester as well as a method for its oxidation to the corresponding methyl ester, and the formation of a new substituted pyrazole under basic conditions.
Keywords: Diazo; carbapenem; pyrazole; β-ketoester.
Figures

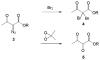



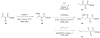

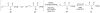
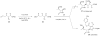
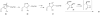
References
-
- Regitz M. Neuere Method. Praep. Org. Chem. 1970;6:76–118.
- Heydt H. In: Science of Synthesis. Padwa A, editor. Vol. 27. Thieme; Stuttgart: 2004. pp. 843–915.
-
- Shih DH, Cama L, Christensen BG. Tetrahedron Lett. 1985;26:587–90.
-
- Espino CG, Fiori KW, Kim M, Du Bois J. J. Am. Chem. Soc. 2004;126:15378–15379. - PubMed
-
- Lee S, Lim H-J, Cha KL, Sulikowski GA. Tetrahedron. 1997;53:16521–16532.
- Chen C, Zhu S-F, Liu B, Wang L-X, Zhou Q-L. J. Am. Chem. Soc. 2007;129:12616–12617. - PubMed
-
- Wang Z, Bi X, Liang Y, Liao P, Dong D. Chem. Commun. 2014;50:3976–3978. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
