Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis
- PMID: 27956794
- PMCID: PMC5124975
- DOI: 10.3748/wjg.v22.i44.9706
Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis
Abstract
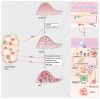
Immune reactivity and chronic low-grade inflammation (metaflammation) play an important role in the pathogenesis of obesity-associated metabolic disorders, including type 2 diabetes and nonalcoholic fatty liver disease (NAFLD), a spectrum of diseases that include liver steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Increased adiposity and insulin resistance contribute to the progression from hepatic steatosis to NASH and fibrosis through the development of proinflammatory and profibrotic processes in the liver, including increased hepatic infiltration of innate and adaptive immune cells, altered balance of cytokines and chemokines, increased reactive oxygen species generation and hepatocellular death. Experimental models of dietary-induced NAFLD/NASH in mice on different genetic backgrounds or knockout mice with different immune reactivity are used for elucidating the pathogenesis of NASH and liver fibrosis. Galectin-3 (Gal-3), a unique chimera-type β-galactoside-binding protein of the galectin family has a regulatory role in immunometabolism and fibrogenesis. Mice deficient in Gal-3 develop pronounced adiposity, hyperglycemia and hepatic steatosis, as well as attenuated liver inflammation and fibrosis when fed an obesogenic high-fat diet. Interleukin (IL)-33, a member of the IL-1 cytokine family, mediates its effects through the ST receptor, which is present on immune and nonimmune cells and participates in immunometabolic and fibrotic disorders. Recent evidence, including our own data, suggests a protective role for the IL-33/IL-33R (ST2) signaling pathway in obesity, adipose tissue inflammation and atherosclerosis, but a profibrotic role in NASH development. The link between Gal-3 and soluble ST2 in myocardial fibrosis and heart failure progression has been demonstrated and we have recently shown that Gal-3 and the IL-33/ST2 pathway interact and both have a profibrotic role in diet-induced NASH. This review discusses the current evidence on the roles of Gal-3 and the IL-33/ST2 pathway and their interplay in obesity-associated hepatic inflammation and fibrogenesis that may be of interest in the development of therapeutic interventions to prevent and/or reverse obesity-associated hepatic inflammation and fibrosis.
Keywords: Galectin-3; Interleukin-33; Liver fibrosis; Nonalcoholic steatohepatitis; ST2.
Conflict of interest statement
Conflict-of-interest statement: No potential conflicts of interest.
Figures

References
-
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. - PubMed
-
- Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, Pastor-Barriuso R, Ahn J, Kim CW, Rampal S, et al. Metabolically Healthy Obesity and the Development of Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2016;111:1133–1140. - PubMed
-
- Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–E591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

