Hepatitis E virus: Western Cape, South Africa
- PMID: 27956810
- PMCID: PMC5124991
- DOI: 10.3748/wjg.v22.i44.9853
Hepatitis E virus: Western Cape, South Africa
Abstract
Aim: To conduct a prospective assessment of anti-hepatitis E virus (HEV) IgG seroprevalence in the Western Cape Province of South Africa in conjunction with evaluating risk factors for exposure.
Methods: Consenting participants attending clinics and wards of Groote Schuur, Red Cross Children's Hospital and their affiliated teaching hospitals in Cape Town, South Africa, were sampled. Healthy adults attending blood donor clinics were also recruited. Patients with known liver disease were excluded and all major ethnic/race groups were included to broadly represent local demographics. Relevant demographic data was captured at the time of sampling using an interviewer-administered confidential questionnaire. Human immunodeficiency virus (HIV) status was self-disclosed. HEV IgG testing was performed using the Wantai® assay.
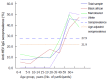
Results: HEV is endemic in the region with a seroprevalence of 27.9% (n = 324/1161) 95%CI: 25.3%-30.5% (21.9% when age-adjusted) with no significant differences between ethnic groups or HIV status. Seroprevalence in children is low but rapidly increases in early adulthood. With univariate analysis, age ≥ 30 years old, pork and bacon/ham consumption suggested risk. In the multivariate analysis, the highest risk factor for HEV IgG seropositivity (OR = 7.679, 95%CI: 5.38-10.96, P < 0.001) was being 30 years or older followed by pork consumption (OR = 2.052, 95%CI: 1.39-3.03, P < 0.001). A recent clinical case demonstrates that HEV genotype 3 may be currently circulating in the Western Cape.
Conclusion: Hepatitis E seroprevalence was considerably higher than previously thought suggesting that hepatitis E warrants consideration in any patient presenting with an unexplained hepatitis in the Western Cape, irrespective of travel history, age or ethnicity.
Keywords: Genotype; Hepatitis E; Pork consumption; Seroprevalence; South Africa.
Conflict of interest statement
Conflict-of-interest statement: Dalton HR has had travel and accommodation costs and consultancy fees from GlaxoSmithKline, Wantai and Gilead; and travel, accommodation and lecture fees from Merck, GFfe Blut GmBh and the Gates foundation. Sonderup M has received travel awards and consultancy fees from AbbVie, Gilead and Roche.
Figures

References
-
- Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. - PubMed
-
- Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–2488. - PubMed
-
- Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. - PubMed
-
- Dalton HR, Hunter JG, Bendall R. Autochthonous hepatitis E in developed countries and HEV/HIV coinfection. Semin Liver Dis. 2013;33:50–61. - PubMed
-
- Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

