A systematic review of orthopaedic manual therapy randomized clinical trials quality
- PMID: 27956817
- PMCID: PMC5125432
- DOI: 10.1080/10669817.2015.1119372
A systematic review of orthopaedic manual therapy randomized clinical trials quality
Abstract
Study Design: Systematic review and meta-analysis. Objectives: To conduct a systematic review and meta-analysis of randomized clinical trials (RCTs) in the orthopaedic manual therapy (OMT) literature from January 2010 to June 2014 in order to determine if the CONSORT checklist and Cochrane Risk of Bias (RoB) assessment tools: (1) are reliable; (2) have improved the reporting and decreased the risk of bias in RCTs in the OMT literature; (3) differ based on journal impact factor (JIF); and (4) scores are associated with each other. Background: The CONSORT statement is used to improve the accuracy of reporting within RCTs. The Cochrane RoB tool was designed to assess the risk of bias within RCTs. To date, no evaluation of the quality of reporting and risk of bias in OMT RCTs has been published. Methods: Relevant RCTs were identified by a literature review from January 2010 to June 2014. The identified RCTs were assessed by two individual reviewers utilizing the 2010 CONSORT checklist and the RoB tool. Agreement and a mean composite total score for each tool were attained in order to determine if the CONSORT and RoB tools were reliable and varied by year and impact factor. Results: A total of 72 RCTs in the OMT literature were identified. A number of categories within the CONSORT and RoB tools demonstrated prevalence-adjusted bias-adjusted kappa (PABAK) scores of less than 0.20 and from 0.20 to 0.40. The total CONSORT and RoB scores were correlated to each other (r = 0.73; 95% CI 0.60 to 0.82; p < 0.0001). There were no statistically significant differences in CONSORT or RoB scores by year. There was a statistically significant correlation between both CONSORT scores and JIF (r = 0.64, 95% CI 0.47 to 0.76; p < 0.0001), and between RoB scores and JIF (r = 0.42, 95% confidence interval 0.21-0.60; p < 0.001). There was not a statistically significant correlation between JIF and year of publication. Conclusion: Our findings suggest that the CONSORT and RoB have a number of items that are unclear and unreliable, and that the quality of reporting in OMT trials has not improved in recent years. Improvements in reporting are necessary to allow advances in OMT practice. Level of Evidence: 1A.
Keywords: CONSORT; Manual therapy; Randomized clinical trails; Risk of bias.
Figures
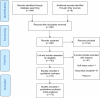


Similar articles
-
The effect of the CONSORT statement on the amount of "unclear" Risk of Bias reporting in Cochrane Systematic Reviews.PLoS One. 2020 Jul 10;15(7):e0235535. doi: 10.1371/journal.pone.0235535. eCollection 2020. PLoS One. 2020. PMID: 32650340 Free PMC article.
-
Quality of reporting and risk of bias: a review of randomised trials in occupational health.Occup Environ Med. 2021 Sep;78(9):691-696. doi: 10.1136/oemed-2020-107038. Epub 2021 Jun 23. Occup Environ Med. 2021. PMID: 34162718 Free PMC article.
-
Impact of the Consolidated Standards of Reporting Trials (CONSORT) checklist on reporting of randomized clinical trials in traditional Chinese medicine.J Evid Based Med. 2015 Nov;8(4):192-208. doi: 10.1111/jebm.12173. J Evid Based Med. 2015. PMID: 26334556
-
Completeness of Reporting Is Suboptimal in Randomized Controlled Trials Published in Rehabilitation Journals, With Trials With Low Risk of Bias Displaying Better Reporting: A Meta-research Study.Arch Phys Med Rehabil. 2022 Sep;103(9):1839-1847. doi: 10.1016/j.apmr.2022.01.156. Epub 2022 Feb 19. Arch Phys Med Rehabil. 2022. PMID: 35192799 Review.
-
Do randomised controlled trials relevant to pharmacy meet best practice standards for quality conduct and reporting? A systematic review.Int J Pharm Pract. 2020 Jun;28(3):220-232. doi: 10.1111/ijpp.12578. Epub 2019 Oct 1. Int J Pharm Pract. 2020. PMID: 31573121
Cited by
-
Reporting quality in preclinical animal experimental research in 2009 and 2018: A nationwide systematic investigation.PLoS One. 2022 Nov 3;17(11):e0275962. doi: 10.1371/journal.pone.0275962. eCollection 2022. PLoS One. 2022. PMID: 36327216 Free PMC article.
-
Disagreements in risk of bias assessment for randomized controlled trials in hypertension-related Cochrane reviews.Trials. 2024 Jun 21;25(1):405. doi: 10.1186/s13063-024-08145-2. Trials. 2024. PMID: 38907276 Free PMC article.
-
Do manual therapies have a specific autonomic effect? An overview of systematic reviews.PLoS One. 2021 Dec 2;16(12):e0260642. doi: 10.1371/journal.pone.0260642. eCollection 2021. PLoS One. 2021. PMID: 34855830 Free PMC article.
-
Is there 'trustworthy' evidence for using manual therapy to treat patients with shoulder dysfunction?: A systematic review.PLoS One. 2024 Jan 18;19(1):e0297234. doi: 10.1371/journal.pone.0297234. eCollection 2024. PLoS One. 2024. PMID: 38236928 Free PMC article.
-
A methodological survey on reporting of pilot and feasibility trials for physiotherapy interventions: a study protocol.BMJ Open. 2019 May 22;9(5):e020580. doi: 10.1136/bmjopen-2017-020580. BMJ Open. 2019. PMID: 31122962 Free PMC article.
References
-
- Savović J, Weeks L, Sterne JA, Turner L, Altman DG, Moher D, et al. . Evaluation of the Cochrane collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3:37.10.1186/2046-4053-3-37 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
