Neoadjuvant Chemoradiotherapy Improving Survival Outcomes for Esophageal Carcinoma: An Updated Meta-analysis
- PMID: 27958230
- PMCID: PMC5198533
- DOI: 10.4103/0366-6999.195464
Neoadjuvant Chemoradiotherapy Improving Survival Outcomes for Esophageal Carcinoma: An Updated Meta-analysis
Abstract
Background: The effectiveness of neoadjuvant chemoradiotherapy (NCRT) treatment for patients with esophageal carcinoma (EC) remains controversial. The aim of this study was to compare the effect of NCRT followed by surgery (NCRTS) with surgery alone (SA) for EC.
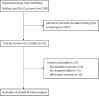
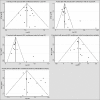
Methods: The PubMed, EMBASE, and the Cochrane Library databases were electronically searched up to August 2015 for all the published studies that investigated EC patients receiving either NCRTS or SA, and the reference lists were also manually examined for the eligible studies. The risk ratio (RR) with 95% confidence intervals (CI s) as effective size was determined to assess the 1-, 3-, 5-year survival rates (SRs), postoperative morbidity, and postoperative mortality. Heterogeneity was determined using the Q-test. The Begg's test and Egger's test were used for assessing any potential publication bias.
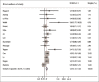
Results: Of 1120 identified studies, 16 eligible studies were included in this analysis (involving 2549 patients). Overall, the pooled results suggested that NCRTS was associated with significantly improved 1-year (RR: 1.07, 95% CI: 1.02-1.13), 3-year (RR: 1.26, 95% CI: 1.14-1.39), and 5-year (RR: 1.36, 95% CI: 1.18-1.56) SRs. However, the results also indicated that NCRTS had no or little effect on postoperative morbidity (RR: 0.93, 95% CI: 0.82-1.05) and postoperative mortality (RR: 1.17, 95% CI: 0.56-2.44).
Conclusions: Compared with SA, NCRTS can increase 1-, 3-, and 5-year SRs in patients with EC.
Conflict of interest statement
There are no conflicts of interest.
Figures




References
-
- Daly JM, Fry WA, Little AG, Winchester DP, McKee RF, Stewart AK, et al. Esophageal cancer: Results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562–72. doi: 10.1016/S1072-7515(00)00238-6. - PubMed
-
- Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101:321–38. doi: 10.1002/bjs.9418. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

