A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates
- PMID: 27958290
- PMCID: PMC5159826
- DOI: 10.1038/ncomms13869
A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates
Abstract
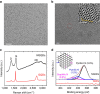
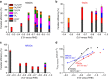
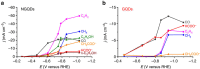
Electroreduction of carbon dioxide into higher-energy liquid fuels and chemicals is a promising but challenging renewable energy conversion technology. Among the electrocatalysts screened so far for carbon dioxide reduction, which includes metals, alloys, organometallics, layered materials and carbon nanostructures, only copper exhibits selectivity towards formation of hydrocarbons and multi-carbon oxygenates at fairly high efficiencies, whereas most others favour production of carbon monoxide or formate. Here we report that nanometre-size N-doped graphene quantum dots (NGQDs) catalyse the electrochemical reduction of carbon dioxide into multi-carbon hydrocarbons and oxygenates at high Faradaic efficiencies, high current densities and low overpotentials. The NGQDs show a high total Faradaic efficiency of carbon dioxide reduction of up to 90%, with selectivity for ethylene and ethanol conversions reaching 45%. The C2 and C3 product distribution and production rate for NGQD-catalysed carbon dioxide reduction is comparable to those obtained with copper nanoparticle-based electrocatalysts.
Figures
References
-
- Whipple D. T. & Kenis P. J. A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 1, 3451–3458 (2010).
-
- Hori Y., Wakebe H., Tsukamoto T. & Koga O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 39, 1833–1839 (1994).
-
- Qiao J., Liu Y., Hong F. & Zhang J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014). - PubMed
-
- Lin S. et al.. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015). - PubMed
-
- Asadi M. et al.. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 5, 4470 (2014). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

