Preparation of Hollow Fe2O3 Nanorods and Nanospheres by Nanoscale Kirkendall Diffusion, and Their Electrochemical Properties for Use in Lithium-Ion Batteries
- PMID: 27958368
- PMCID: PMC5153625
- DOI: 10.1038/srep38933
Preparation of Hollow Fe2O3 Nanorods and Nanospheres by Nanoscale Kirkendall Diffusion, and Their Electrochemical Properties for Use in Lithium-Ion Batteries
Abstract
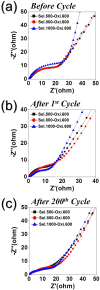
A novel process for the preparation of aggregate-free metal oxide nanopowders with spherical (0D) and non-spherical (1D) hollow nanostructures was introduced. Carbon nanofibers embedded with iron selenide (FeSe) nanopowders with various nanostructures are prepared via the selenization of electrospun nanofibers. Ostwald ripening occurs during the selenization process, resulting in the formation of a FeSe-C composite nanofiber exhibiting a hierarchical structure. These nanofibers transform into aggregate-free hollow Fe2O3 powders via the complete oxidation of FeSe and combustion of carbon. Indeed, the zero- (0D) and one-dimensional (1D) FeSe nanocrystals transform into the hollow-structured Fe2O3 nanopowders via a nanoscale Kirkendall diffusion process, thus conserving their overall morphology. The discharge capacities for the 1000th cycle of the hollow-structured Fe2O3 nanopowders obtained from the FeSe-C composite nanofibers prepared at selenization temperatures of 500, 800, and 1000 °C at a current density of 1 A g-1 are 932, 767, and 544 mA h g-1, respectively; and their capacity retentions from the second cycle are 88, 92, and 78%, respectively. The high structural stabilities of these hollow Fe2O3 nanopowders during repeated lithium insertion/desertion processes result in superior lithium-ion storage performances.
Figures
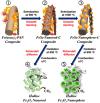
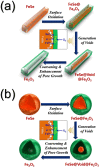

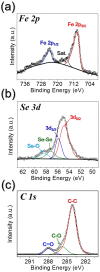

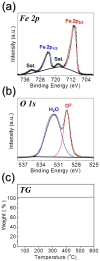


References
-
- Wu R. et al.. Porous spinel ZnxCo3–xO4 hollow polyhedra templated for high-rate lithium-ion batteries. ACS Nano 8, 6297–6303 (2014). - PubMed
-
- Yu L., Xia B. Y., Wang X. & Lou X. W. General formation of M–MoS3 (M=Co, Ni) hollow structures with enhanced electrocatalytic activity for hydrogen evolution. Adv. Mater. 28, 92–97 (2016). - PubMed
-
- Ji L. et al.. Controlling SEI formation on SnSb‐porous carbon nanofibers for improved Na ion storage. Adv. Mater. 26, 2901–2908 (2014). - PubMed
-
- Niu C. et al.. VO2 nanowires assembled into hollow microspheres for high-rate and long-life lithium batteries. Nano Lett. 14, 2873–2878 (2014). - PubMed
-
- Liu J. et al.. Three-dimensionally interconnected nickel–antimony intermetallic hollow nanospheres as anode material for high-rate sodium-ion batteries. Nano Energy 16, 389–398 (2015).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

