Zika Virus RNA Replication and Persistence in Brain and Placental Tissue
- PMID: 27959260
- PMCID: PMC5382738
- DOI: 10.3201/eid2303.161499
Zika Virus RNA Replication and Persistence in Brain and Placental Tissue
Abstract
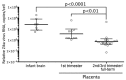
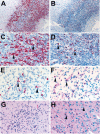
Zika virus is causally linked with congenital microcephaly and may be associated with pregnancy loss. However, the mechanisms of Zika virus intrauterine transmission and replication and its tropism and persistence in tissues are poorly understood. We tested tissues from 52 case-patients: 8 infants with microcephaly who died and 44 women suspected of being infected with Zika virus during pregnancy. By reverse transcription PCR, tissues from 32 (62%) case-patients (brains from 8 infants with microcephaly and placental/fetal tissues from 24 women) were positive for Zika virus. In situ hybridization localized replicative Zika virus RNA in brains of 7 infants and in placentas of 9 women who had pregnancy losses during the first or second trimester. These findings demonstrate that Zika virus replicates and persists in fetal brains and placentas, providing direct evidence of its association with microcephaly. Tissue-based reverse transcription PCR extends the time frame of Zika virus detection in congenital and pregnancy-associated infections.
Keywords: RT-PCR; Zika virus; brain; formalin-fixed; in-situ hybridization; paraffin-embedded tissues; placenta; replication; vector-borne infections; viruses.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

