Microtubule actin cross-linking factor 1, a novel target in glioblastoma
- PMID: 27959385
- PMCID: PMC6903898
- DOI: 10.3892/ijo.2016.3798
Microtubule actin cross-linking factor 1, a novel target in glioblastoma
Abstract
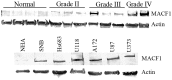
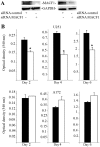
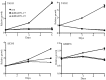
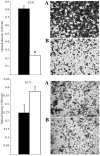
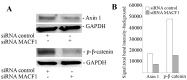
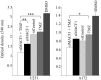
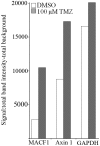
Genetic heterogeneity is recognized as a major contributing factor of glioblastoma resistance to clinical treatment modalities and consequently low overall survival rates. This genetic diversity results in variations in protein expression, both intratumorally and between individual glioblastoma patients. In this regard, the spectraplakin protein, microtubule actin cross-linking factor 1 (MACF1), was examined in glioblastoma. An expression analysis of MACF1 in various types of brain tumor tissue revealed that MACF1 was predominately present in grade III-IV astroctyomas and grade IV glioblastoma, but not in normal brain tissue, normal human astrocytes and lower grade brain tumors. Subsequent genetic inhibition experiments showed that suppression of MACF1 selectively inhibited glioblastoma cell proliferation and migration in cell lines established from patient derived xenograft mouse models and immortalized glioblastoma cell lines that were associated with downregulation of the Wnt-signaling mediators, Axin1 and β-catenin. Additionally, concomitant MACF1 silencing with the chemotherapeutic agent temozolomide (TMZ) used for the clinical treatment of glioblastomas cooperatively reduced the proliferative capacity of glioblastoma cells. In conclusion, the present study represents the first investigation on the functional role of MACF1 in tumor cell biology, as well as demonstrates its potential as a unique biomarker that can be targeted synergistically with TMZ as part of a combinatorial therapeutic approach for the treatment of genetically multifarious glioblastomas.
Figures

References
-
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

