Humanized Mouse Models of Clinical Disease
- PMID: 27959627
- PMCID: PMC5280554
- DOI: 10.1146/annurev-pathol-052016-100332
Humanized Mouse Models of Clinical Disease
Abstract
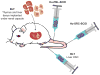
Immunodeficient mice engrafted with functional human cells and tissues, that is, humanized mice, have become increasingly important as small, preclinical animal models for the study of human diseases. Since the description of immunodeficient mice bearing mutations in the IL2 receptor common gamma chain (IL2rgnull) in the early 2000s, investigators have been able to engraft murine recipients with human hematopoietic stem cells that develop into functional human immune systems. These mice can also be engrafted with human tissues such as islets, liver, skin, and most solid and hematologic cancers. Humanized mice are permitting significant progress in studies of human infectious disease, cancer, regenerative medicine, graft-versus-host disease, allergies, and immunity. Ultimately, use of humanized mice may lead to the implementation of truly personalized medicine in the clinic. This review discusses recent progress in the development and use of humanized mice and highlights their utility for the study of human diseases.
Keywords: allergy; autoimmunity; cancer; humanized mice; immunodeficient mice; infectious disease; regenerative medicine.
Figures

References
-
- Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34:433–54. - PubMed
-
- Brehm MA, Bortell R, Verma M, Shultz LD, Greiner DL. Humanized Mice in Translational Immunology. In: Tan SL, editor. Translational Immunology: Mechanisms and Pharmacological Approaches. Elsevier; 2016. pp. 285–326.
-
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

