Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease
- PMID: 27959701
- PMCID: PMC5481200
- DOI: 10.1056/NEJMoa1611770
Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease
Abstract
Background: The up-regulation of P-selectin in endothelial cells and platelets contributes to the cell-cell interactions that are involved in the pathogenesis of vaso-occlusion and sickle cell-related pain crises. The safety and efficacy of crizanlizumab, an antibody against the adhesion molecule P-selectin, were evaluated in patients with sickle cell disease.
Methods: In this double-blind, randomized, placebo-controlled, phase 2 trial, we assigned patients to receive low-dose crizanlizumab (2.5 mg per kilogram of body weight), high-dose crizanlizumab (5.0 mg per kilogram), or placebo, administered intravenously 14 times over a period of 52 weeks. Patients who were receiving concomitant hydroxyurea as well as those not receiving hydroxyurea were included in the study. The primary end point was the annual rate of sickle cell-related pain crises with high-dose crizanlizumab versus placebo. The annual rate of days hospitalized, the times to first and second crises, annual rates of uncomplicated crises (defined as crises other than the acute chest syndrome, hepatic sequestration, splenic sequestration, or priapism) and the acute chest syndrome, and patient-reported outcomes were also assessed.
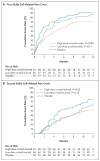
Results: A total of 198 patients underwent randomization at 60 sites. The median rate of crises per year was 1.63 with high-dose crizanlizumab versus 2.98 with placebo (indicating a 45.3% lower rate with high-dose crizanlizumab, P=0.01). The median time to the first crisis was significantly longer with high-dose crizanlizumab than with placebo (4.07 vs. 1.38 months, P=0.001), as was the median time to the second crisis (10.32 vs. 5.09 months, P=0.02). The median rate of uncomplicated crises per year was 1.08 with high-dose crizanlizumab, as compared with 2.91 with placebo (indicating a 62.9% lower rate with high-dose crizanlizumab, P=0.02). Adverse events that occurred in 10% or more of the patients in either active-treatment group and at a frequency that was at least twice as high as that in the placebo group were arthralgia, diarrhea, pruritus, vomiting, and chest pain.
Conclusions: In patients with sickle cell disease, crizanlizumab therapy resulted in a significantly lower rate of sickle cell-related pain crises than placebo and was associated with a low incidence of adverse events. (Funded by Selexys Pharmaceuticals and others; SUSTAIN ClinicalTrials.gov number, NCT01895361 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Go with the Flow.N Engl J Med. 2017 Feb 2;376(5):485-487. doi: 10.1056/NEJMe1615651. N Engl J Med. 2017. PMID: 28146663 No abstract available.
-
Unclogging sickle cell anaemia.Nat Rev Mol Cell Biol. 2017 Apr;18(4):214. doi: 10.1038/nrm.2017.17. Epub 2017 Mar 1. Nat Rev Mol Cell Biol. 2017. PMID: 28248321 No abstract available.
-
Crizanlizumab in Sickle Cell Disease.N Engl J Med. 2017 May 4;376(18):1795-1796. doi: 10.1056/NEJMc1703162. N Engl J Med. 2017. PMID: 28467873 No abstract available.
-
[A new therapeutic era in sickle cell disease].Rev Med Interne. 2017 Sep;38(9):569-571. doi: 10.1016/j.revmed.2017.05.006. Epub 2017 Jun 16. Rev Med Interne. 2017. PMID: 28624233 French. No abstract available.
References
-
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. - PubMed
-
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease — life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–44. - PubMed
-
- Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–56. - PubMed
-
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases