Histological Transformation and Progression in Follicular Lymphoma: A Clonal Evolution Study
- PMID: 27959929
- PMCID: PMC5154502
- DOI: 10.1371/journal.pmed.1002197
Histological Transformation and Progression in Follicular Lymphoma: A Clonal Evolution Study
Abstract
Background: Follicular lymphoma (FL) is an indolent, yet incurable B cell malignancy. A subset of patients experience an increased mortality rate driven by two distinct clinical end points: histological transformation and early progression after immunochemotherapy. The nature of tumor clonal dynamics leading to these clinical end points is poorly understood, and previously determined genetic alterations do not explain the majority of transformed cases or accurately predict early progressive disease. We contend that detailed knowledge of the expansion patterns of specific cell populations plus their associated mutations would provide insight into therapeutic strategies and disease biology over the time course of FL clinical histories.
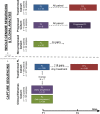
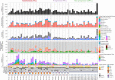
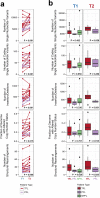
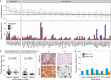
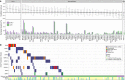
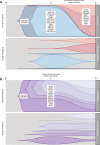
Methods and findings: Using a combination of whole genome sequencing, targeted deep sequencing, and digital droplet PCR on matched diagnostic and relapse specimens, we deciphered the constituent clonal populations in 15 transformation cases and 6 progression cases, and measured the change in clonal population abundance over time. We observed widely divergent patterns of clonal dynamics in transformed cases relative to progressed cases. Transformation specimens were generally composed of clones that were rare or absent in diagnostic specimens, consistent with dramatic clonal expansions that came to dominate the transformation specimens. This pattern was independent of time to transformation and treatment modality. By contrast, early progression specimens were composed of clones that were already present in the diagnostic specimens and exhibited only moderate clonal dynamics, even in the presence of immunochemotherapy. Analysis of somatic mutations impacting 94 genes was undertaken in an extension cohort consisting of 395 samples from 277 patients in order to decipher disrupted biology in the two clinical end points. We found 12 genes that were more commonly mutated in transformed samples than in the preceding FL tumors, including TP53, B2M, CCND3, GNA13, S1PR2, and P2RY8. Moreover, ten genes were more commonly mutated in diagnostic specimens of patients with early progression, including TP53, BTG1, MKI67, and XBP1.
Conclusions: Our results illuminate contrasting modes of evolution shaping the clinical histories of transformation and progression. They have implications for interpretation of evolutionary dynamics in the context of treatment-induced selective pressures, and indicate that transformation and progression will require different clinical management strategies.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: SPS is a founder and shareholder of Contextual Genomics Inc., developer of clinical genomic tests for cancer.
Figures




References
-
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909–18. - PubMed
-
- Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9(7):717–20. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

