Transfusion: -80°C Frozen Blood Products Are Safe and Effective in Military Casualty Care
- PMID: 27959967
- PMCID: PMC5154589
- DOI: 10.1371/journal.pone.0168401
Transfusion: -80°C Frozen Blood Products Are Safe and Effective in Military Casualty Care
Abstract
Introduction: The Netherlands Armed Forces use -80°C frozen red blood cells (RBCs), plasma and platelets combined with regular liquid stored RBCs, for the treatment of (military) casualties in Medical Treatment Facilities abroad. Our objective was to assess and compare the use of -80°C frozen blood products in combination with the different transfusion protocols and their effect on the outcome of trauma casualties.
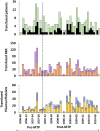
Materials and methods: Hemovigilance and combat casualties data from Afghanistan 2006-2010 for 272 (military) trauma casualties with or without massive transfusions (MT: ≥6 RBC/24hr, N = 82 and non-MT: 1-5 RBC/24hr, N = 190) were analyzed retrospectively. In November 2007, a massive transfusion protocol (MTP; 4:3:1 RBC:Plasma:Platelets) for ATLS® class III/IV hemorrhage was introduced in military theatre. Blood product use, injury severity and mortality were assessed pre- and post-introduction of the MTP. Data were compared to civilian and military trauma studies to assess effectiveness of the frozen blood products and MTP.
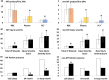
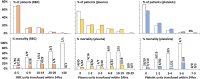
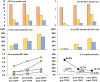
Results: No ABO incompatible blood products were transfused and only 1 mild transfusion reaction was observed with 3,060 transfused products. In hospital mortality decreased post-MTP for MT patients from 44% to 14% (P = 0.005) and for non-MT patients from 12.7% to 5.9% (P = 0.139). Average 24-hour RBC, plasma and platelet ratios were comparable and accompanying 24-hour mortality rates were low compared to studies that used similar numbers of liquid stored (and on site donated) blood products.
Conclusion: This report describes for the first time that the combination of -80°C frozen platelets, plasma and red cells is safe and at least as effective as standard blood products in the treatment of (military) trauma casualties. Frozen blood can save the lives of casualties of armed conflict without the need for in-theatre blood collection. These results may also contribute to solutions for logistic problems in civilian blood supply in remote areas.
Conflict of interest statement
The authors declare that there are no conflicts of interest that could inappropriately influence (bias) their work.
Figures
References
-
- Hakre S, Peel SA, O'Connell RJ, Sanders-Buell EE, Jagodzinski LL, Eggleston JC, et al. Transfusion-transmissible viral infections among US military recipients of whole blood and platelets during Operation Enduring Freedom and Operation Iraqi Freedom. Transfusion. 2011;51(3):473–85. 10.1111/j.1537-2995.2010.02906.x - DOI - PubMed
-
- Strandenes G, Berseus O, Cap AP, Hervig T, Reade M, Prat N, et al. Low titer group O whole blood in emergency situations. Shock. 2014;41 Suppl 1:70–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

