Intravital Förster resonance energy transfer imaging reveals osteopontin-mediated polymorphonuclear leukocyte activation by tumor cell emboli
- PMID: 27960041
- PMCID: PMC5329161
- DOI: 10.1111/cas.13132
Intravital Förster resonance energy transfer imaging reveals osteopontin-mediated polymorphonuclear leukocyte activation by tumor cell emboli
Abstract
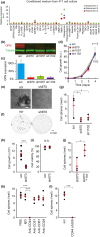
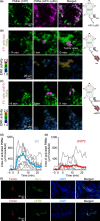
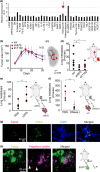
Myeloid-derived suppressor cells (MDSCs) cause paraneoplastic leukemoid reactions and facilitate tumor cell metastasis. However, the interaction of MDSCs with tumor cells in live tissue has not been adequately visualized. To accomplish this task, we developed an intravital imaging protocol to observe metastasized tumor cells in mouse lungs. For visualization of the activation of MDSCs, bone marrow cells derived from transgenic mice expressing a Förster resonance energy transfer biosensor for ERK were implanted into host mice. Under a two-photon excitation microscope, numerous polymorphonuclear cells (PMNs) were found to infiltrate the lungs of tumor-bearing mice in which 4T1 mammary tumor cells were implanted into the footpads. By Förster resonance energy transfer imaging, we found ERK activation in PMNs around the 4T1 tumor emboli in the lungs. Because antibody array analysis implied the involvement of osteopontin (OPN) in the metastasis of 4T1 cells, we further analyzed the effect of OPN knockdown. The OPN knockdown in 4T1 cells did not affect the cell growth, but markedly suppressed lung metastasis of 4T1 cells and ERK activation in PMNs in the lung. Intravenous injection of recombinant OPN restored the lung metastasis of OPN-deficient 4T1 cells, suggesting that OPN functioned in a paracrine manner. It has been reported that ERK activation of neutrophils causes NETosis and that PMNs promote metastasis of tumor cells by NETosis. In agreement with previous reports, the NETosis inhibitor DNase I inhibited lung metastasis of 4T1 cells. These observations suggest that OPN promotes metastasis of 4T1 cells by activating PMNs and inducing NETosis.
Keywords: Cancer imaging; immune system imaging; immunosurveillance; tumor immunology; two-photon excitation microscopy.
© 2016 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
ERK Activity Imaging During Migration of Living Cells In Vitro and In Vivo.Int J Mol Sci. 2019 Feb 5;20(3):679. doi: 10.3390/ijms20030679. Int J Mol Sci. 2019. PMID: 30764494 Free PMC article. Review.
-
Serum osteopontin, an enhancer of tumor metastasis to bone, promotes B16 melanoma cell migration.J Cell Biochem. 2007 Jul 1;101(4):979-86. doi: 10.1002/jcb.21298. J Cell Biochem. 2007. PMID: 17390343
-
Bone marrow-derived, alternatively activated macrophages enhance solid tumor growth and lung metastasis of mammary carcinoma cells in a Balb/C mouse orthotopic model.Breast Cancer Res. 2012 May 22;14(3):R81. doi: 10.1186/bcr3195. Breast Cancer Res. 2012. PMID: 22616919 Free PMC article.
-
Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells.Carcinogenesis. 2006 Jun;27(6):1134-45. doi: 10.1093/carcin/bgi352. Epub 2006 Feb 12. Carcinogenesis. 2006. PMID: 16474180
-
Role of the metastasis-promoting protein osteopontin in the tumour microenvironment.J Cell Mol Med. 2010 Aug;14(8):2037-44. doi: 10.1111/j.1582-4934.2010.01115.x. Epub 2010 Jul 1. J Cell Mol Med. 2010. PMID: 20597997 Free PMC article. Review.
Cited by
-
ERK Activity Imaging During Migration of Living Cells In Vitro and In Vivo.Int J Mol Sci. 2019 Feb 5;20(3):679. doi: 10.3390/ijms20030679. Int J Mol Sci. 2019. PMID: 30764494 Free PMC article. Review.
-
Tumor-associated neutrophils (TANs) in human carcinoma-draining lymph nodes: a novel TAN compartment.Clin Transl Immunology. 2021 Feb 16;10(2):e1252. doi: 10.1002/cti2.1252. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33643653 Free PMC article.
-
Cortical Actin Dynamics in Endothelial Permeability.Curr Top Membr. 2018;82:141-195. doi: 10.1016/bs.ctm.2018.09.003. Epub 2018 Oct 15. Curr Top Membr. 2018. PMID: 30360779 Free PMC article.
-
Neutrophil dynamics in the tumor microenvironment.J Clin Invest. 2021 Mar 15;131(6):e143759. doi: 10.1172/JCI143759. J Clin Invest. 2021. PMID: 33720040 Free PMC article. Review.
-
Decreased Lung Metastasis in Triple Negative Breast Cancer Following Locally Delivered Supratherapeutic Paclitaxel-Loaded Polyglycerol Carbonate Nanoparticle Therapy.Biomacromolecules. 2024 Mar 11;25(3):1800-1809. doi: 10.1021/acs.biomac.3c01258. Epub 2024 Feb 21. Biomacromolecules. 2024. PMID: 38380618 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

