Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis
- PMID: 27960084
- PMCID: PMC5157696
- DOI: 10.1016/j.ccell.2016.10.009
Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis
Abstract
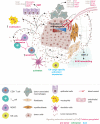
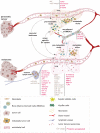
Tumor-secreted extracellular vesicles (EVs) are critical mediators of intercellular communication between tumor cells and stromal cells in local and distant microenvironments. Accordingly, EVs play an essential role in both primary tumor growth and metastatic evolution. EVs orchestrate multiple systemic pathophysiological processes, such as coagulation, vascular leakiness, and reprogramming of stromal recipient cells to support pre-metastatic niche formation and subsequent metastasis. Clinically, EVs may be biomarkers and novel therapeutic targets for cancer progression, particularly for predicting and preventing future metastatic development.
Keywords: cancer; exosomes; extracellular vesicles; intercellular communication; metastasis; microvesicles; pre-metastatic niche.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
References
-
- Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood cells, molecules & diseases. 2005;35:169–173. - PubMed
-
- Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–1424. - PubMed
-
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008;10:619–624. - PubMed
-
- Alegre E, Sanmamed MF, Rodriguez C, Carranza O, Martin-Algarra S, Gonzalez A. Study of circulating microRNA-125b levels in serum exosomes in advanced melanoma. Archives of pathology & laboratory medicine. 2014;138:828–832. - PubMed
-
- Alegre E, Zubiri L, Perez-Gracia JL, Gonzalez-Cao M, Soria L, Martin-Algarra S, Gonzalez A. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clinica chimica acta; international journal of clinical chemistry. 2016;454:28–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
