Critical Role of the CXCL10/C-X-C Chemokine Receptor 3 Axis in Promoting Leukocyte Recruitment and Neuronal Injury during Traumatic Optic Neuropathy Induced by Optic Nerve Crush
- PMID: 27960090
- PMCID: PMC5389365
- DOI: 10.1016/j.ajpath.2016.10.009
Critical Role of the CXCL10/C-X-C Chemokine Receptor 3 Axis in Promoting Leukocyte Recruitment and Neuronal Injury during Traumatic Optic Neuropathy Induced by Optic Nerve Crush
Abstract
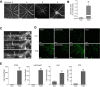
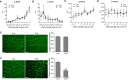
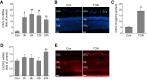
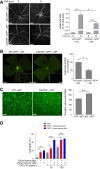
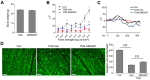
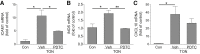
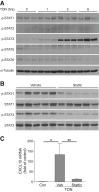
Traumatic optic neuropathy (TON) is an acute injury of the optic nerve secondary to trauma. Loss of retinal ganglion cells (RGCs) is a key pathological process in TON, yet mechanisms responsible for RGC death remain unclear. In a mouse model of TON, real-time noninvasive imaging revealed a dramatic increase in leukocyte rolling and adhesion in veins near the optic nerve (ON) head at 9 hours after ON injury. Although RGC dysfunction and loss were not detected at 24 hours after injury, massive leukocyte infiltration was observed in the superficial retina. These cells were identified as T cells, microglia/monocytes, and neutrophils but not B cells. CXCL10 is a chemokine that recruits leukocytes after binding to its receptor C-X-C chemokine receptor (CXCR) 3. The levels of CXCL10 and CXCR3 were markedly elevated in TON, and up-regulation of CXCL10 was mediated by STAT1/3. Deleting CXCR3 in leukocytes significantly reduced leukocyte recruitment, and prevented RGC death at 7 days after ON injury. Treatment with CXCR3 antagonist attenuated TON-induced RGC dysfunction and cell loss. In vitro co-culture of primary RGCs with leukocytes resulted in increased RGC apoptosis, which was exaggerated in the presence of CXCL10. These results indicate that leukocyte recruitment in retinal vessels near the ON head is an early event in TON and the CXCL10/CXCR3 axis has a critical role in recruiting leukocytes and inducing RGC death.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Caspase-7: a critical mediator of optic nerve injury-induced retinal ganglion cell death.Mol Neurodegener. 2015 Aug 26;10:40. doi: 10.1186/s13024-015-0039-2. Mol Neurodegener. 2015. PMID: 26306916 Free PMC article.
-
Endoplasmic reticulum stress-regulated CXCR3 pathway mediates inflammation and neuronal injury in acute glaucoma.Cell Death Dis. 2015 Oct 8;6(10):e1900. doi: 10.1038/cddis.2015.281. Cell Death Dis. 2015. PMID: 26448323 Free PMC article.
-
AAV2-mediated GRP78 Transfer Alleviates Retinal Neuronal Injury by Downregulating ER Stress and Tau Oligomer Formation.Invest Ophthalmol Vis Sci. 2018 Sep 4;59(11):4670-4682. doi: 10.1167/iovs.18-24427. Invest Ophthalmol Vis Sci. 2018. PMID: 30267089 Free PMC article.
-
Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy.Front Immunol. 2022 Mar 4;13:860070. doi: 10.3389/fimmu.2022.860070. eCollection 2022. Front Immunol. 2022. PMID: 35309305 Free PMC article. Review.
-
Role of HDACs in optic nerve damage-induced nuclear atrophy of retinal ganglion cells.Neurosci Lett. 2016 Jun 20;625:11-5. doi: 10.1016/j.neulet.2015.12.012. Epub 2015 Dec 28. Neurosci Lett. 2016. PMID: 26733303 Free PMC article. Review.
Cited by
-
Platelet factor 4 promotes rapid replication and propagation of Dengue and Japanese encephalitis viruses.EBioMedicine. 2019 Jan;39:332-347. doi: 10.1016/j.ebiom.2018.11.049. Epub 2018 Dec 5. EBioMedicine. 2019. PMID: 30527622 Free PMC article.
-
Lipoxins A4 and B4 inhibit glial cell activation via CXCR3 signaling in acute retinal neuroinflammation.J Neuroinflammation. 2024 Jan 11;21(1):18. doi: 10.1186/s12974-024-03010-0. J Neuroinflammation. 2024. PMID: 38212822 Free PMC article.
-
TREM2 deficiency in microglia accelerates photoreceptor cell death and immune cell infiltration following retinal detachment.Cell Death Dis. 2023 Mar 28;14(3):219. doi: 10.1038/s41419-023-05735-x. Cell Death Dis. 2023. PMID: 36977680 Free PMC article.
-
Neuroprotection of SRT2104 in Murine Ischemia/Reperfusion Injury Through the Enhancement of Sirt1-Mediated Deacetylation.Invest Ophthalmol Vis Sci. 2023 Apr 3;64(4):31. doi: 10.1167/iovs.64.4.31. Invest Ophthalmol Vis Sci. 2023. PMID: 37099021 Free PMC article.
-
Optic Nerve Crush Does Not Induce Retinal Ganglion Cell Loss in the Contralateral Eye.Invest Ophthalmol Vis Sci. 2025 Mar 3;66(3):49. doi: 10.1167/iovs.66.3.49. Invest Ophthalmol Vis Sci. 2025. PMID: 40126507 Free PMC article.
References
-
- Ahmad S., Fatteh N., El-Sherbiny N.M., Naime M., Ibrahim A.S., El-Sherbini A.M., El-Shafey S.A., Khan S., Fulzele S., Gonzales J., Liou G.I. Potential role of A2A adenosine receptor in traumatic optic neuropathy. J Neuroimmunol. 2013;264:54–64. - PubMed
-
- Furtado J.M., Lansingh V.C., Carter M.J., Milanese M.F., Pena B.N., Ghersi H.A., Bote P.L., Nano M.E., Silva J.C. Causes of blindness and visual impairment in Latin America. Surv Ophthalmol. 2012;57:149–177. - PubMed
-
- Sarkies N. Traumatic optic neuropathy. Eye (Lond) 2004;18:1122–1125. - PubMed
-
- Wu N., Yin Z.Q., Wang Y. Traumatic optic neuropathy therapy: an update of clinical and experimental studies. J Int Med Res. 2008;36:883–889. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

