Differential gene and protein expression of chemokines and cytokines in synovial fluid of patients with arthritis
- PMID: 27964744
- PMCID: PMC5154157
- DOI: 10.1186/s13075-016-1196-6
Differential gene and protein expression of chemokines and cytokines in synovial fluid of patients with arthritis
Abstract
Background: Psoriatic arthritis (PsA), an inflammatory musculoskeletal disease, develops in approximately 30% of patients with psoriasis. Previously, chemokine (C-X-C motif) ligand 10 (CXCL10) was identified as a predictive biomarker of PsA in patients with psoriasis and was reduced after development of PsA. The purpose of the present study was to explore messenger RNA (mRNA) and protein expression of CXCL10 and its receptor, chemokine (C-X-C motif) receptor 3 (CXCR3), in the joints of patients with PsA to gain insight into their role in the pathogenesis of the disease.
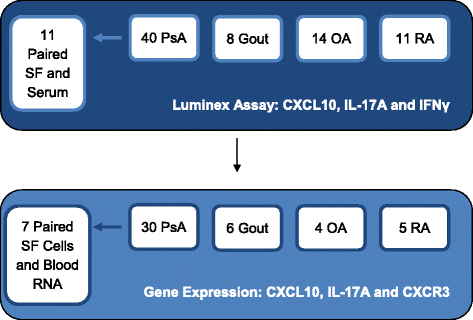
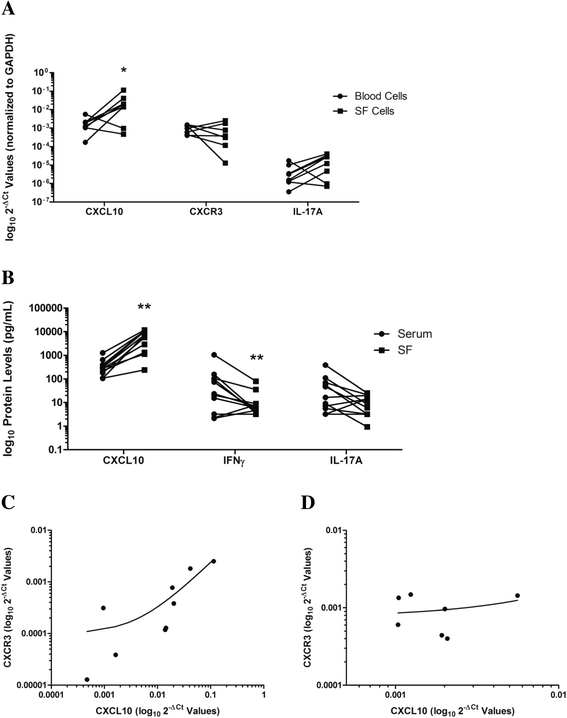
Methods: Sera from 47 patients with PsA and 33 healthy control subjects were compared for expression of CXCL10 by Luminex assay. Synovial fluid (SF) was obtained from patients with PsA (n = 40), osteoarthritis (OA; n = 14), gout (n = 8), and rheumatoid arthritis (RA; n = 11) during clinical care. SF mRNA and protein expression of CXCL10, interleukin-17A (IL-17A), CXCR3, TBX21, RORC and/or interferon γ (IFNγ) were compared among the above-mentioned disease groups, as well as in paired SF and serum samples from patients with PsA using real-time polymerase chain reaction and Luminex assays, respectively.
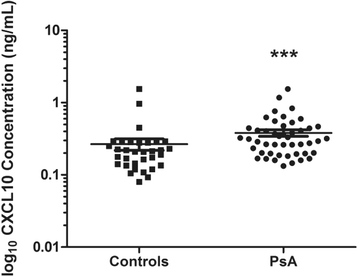
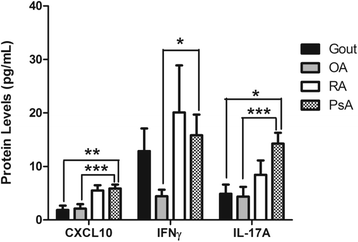
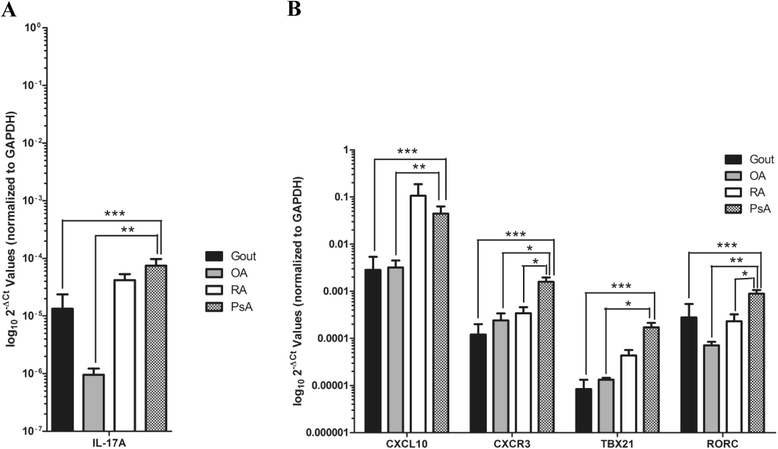
Results: Serum CXCL10 was significantly higher in patients with PsA than in control subjects (p = 0.0007). CXCL10, IL-17A, and TBX21 expression were elevated in SF cells of patients with PsA compared with those of patients with OA and gout, but not those of patients with RA. CXCR3 and RORC were elevated in PsA SF cells compared with all other patient groups. Concordant results were obtained for CXCL10 and IL-17A protein expression. IFNγ was elevated in PsA SF compared with OA SF (p = 0.015). CXCL10 protein expression was substantially increased in SF (median 7283.9 pg/ml, interquartile range [IQR] 1330-10,362 pg/ml) compared with paired serum samples (median 282.06, IQR 180.7-395.8 pg/ml; p = 0.001), whereas IFNγ was significantly reduced (SF median 6.03 pg/ml, IQR 4.47-8.94 pg/ml; versus serum median 23.70 pg/ml, IQR 3.2-104.6 pg/ml; p = 0.001).
Conclusions: CXCL10 may have an important etiological role in PsA that is analogous to that in RA, and it is a candidate biomarker to distinguish PsA from healthy individuals and from patients with OA and gout.
Keywords: Biomarkers; Chemokines; Cytokines; Psoriatic arthritis; Synovial cells; Synovial fluid.
Figures
References
-
- Christophers E, Barker JN, Griffiths CE, Dauden E, Milligan G, Molta C, et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. J Eur Acad Dermatol Venereol. 2010;24:548–54. doi: 10.1111/j.1468-3083.2009.03463.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

