Retrospective cohort study of usage patterns of epidural injections for spinal pain in the US fee-for-service Medicare population from 2000 to 2014
- PMID: 27965254
- PMCID: PMC5168679
- DOI: 10.1136/bmjopen-2016-013042
Retrospective cohort study of usage patterns of epidural injections for spinal pain in the US fee-for-service Medicare population from 2000 to 2014
Abstract
Objective: To assess the usage patterns of epidural injections for chronic spinal pain in the fee-for-service (FFS) Medicare population from 2000 to 2014 in the USA.
Design: A retrospective cohort.
Methods: The descriptive analysis of the administrative database from Centers for Medicare and Medicaid Services (CMS) Physician/Supplier Procedure Summary (PSPS) master data from 2000 to 2014 was performed. The guidance from Strengthening the Reporting of Observational studies in Epidemiology (STROBE) was applied. Analysis included multiple variables based on the procedures, specialties and geography.
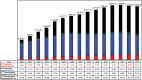
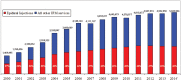
Results: Overall epidural injections increased 99% per 100 000 Medicare beneficiaries with an annual increase of 5% from 2000 to 2014. Lumbar interlaminar and caudal epidural injections constituted 36.2% of all epidural injections, with an overall decrease of 2% and an annual decrease of 0.2% per 100 000 Medicare beneficiaries. However, lumbosacral transforaminal epidural injections increased 609% with an annual increase of 15% from 2000 to 2014 per 100 000 Medicare population.
Conclusions: Usage of epidural injections increased from 2000 to 2014, with a decline thereafter. However, an escalating growth has been seen for lumbosacral transforaminal epidural injections despite numerous reports of complications and regulations to curb the usage of transforaminal epidural injections.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
LM has provided limited consulting services to Semnur Pharmaceuticals, Incorporated, which is developing non-particulate steroids. JAH is a consultant for Medtronic.
Figures
References
-
- US Food and Drug Administration. Drug Safety Communications. FDA Drug Safety Communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM394286.pdf (accessed 22 Mar 2016).
-
- Food and Drug Administration. Anesthetic and Analgesic Drug Products Advisory Committee Meeting. November 24–25, 2014. Epidural steroid injections (ESI) and the risk of serious neurologic adverse reactions. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria... (accessed 22 Mar 2016).
-
- Manchikanti L, Candido KD, Singh V et al. Epidural steroid warning controversy still dogging FDA. Pain Physician 2014;17:E451–74. - PubMed
-
- Manchikanti L, Falco FJE, Benyamin RM et al. Epidural steroid injections safety recommendations by the Multi-Society Pain Workgroup (MPW): more regulations without evidence or clarification. Pain Physician 2014;17:E575–88. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical