Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren's syndrome
- PMID: 27965259
- PMCID: PMC5530351
- DOI: 10.1136/annrheumdis-2016-210448
Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren's syndrome
Abstract
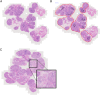
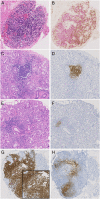
Labial salivary gland (LSG) biopsy is used in the classification of primary Sjögren's syndrome (PSS) and in patient stratification in clinical trials. It may also function as a biomarker. The acquisition of tissue and histological interpretation is variable and needs to be standardised for use in clinical trials. A modified European League Against Rheumatism consensus guideline development strategy was used. The steering committee of the ad hoc working group identified key outstanding points of variability in LSG acquisition and analysis. A 2-day workshop was held to develop consensus where possible and identify points where further discussion/data was needed. These points were reviewed by a subgroup of experts on PSS histopathology and then circulated via an online survey to 50 stakeholder experts consisting of rheumatologists, histopathologists and oral medicine specialists, to assess level of agreement (0-10 scale) and comments. Criteria for agreement were a mean score ≥6/10 and 75% of respondents scoring ≥6/10. Thirty-nine (78%) experts responded and 16 points met criteria for agreement. These points are focused on tissue requirements, identification of the characteristic focal lymphocytic sialadenitis, calculation of the focus score, identification of germinal centres, assessment of the area of leucocyte infiltration, reporting standards and use of prestudy samples for clinical trials. We provide standardised consensus guidance for the use of labial salivary gland histopathology in the classification of PSS and in clinical trials and identify areas where further research is required to achieve evidence-based consensus.
Keywords: Autoimmunity; Outcomes research; Sjøgren's Syndrome.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: SJB has received honoraria/consultancy fees in the field of Sjögren's syndrome in 2015–2016 for AstraZeneca, Celgene, Glenmark, Eli Lilly, Novartis, Ono and UCB Pharmaceuticals. Roche provided rituximab for the TRACTISS study. BAF has received honoraria/consultancy fees from Novartis, Roche and Medimmune. FB has received honoraria/consultancy fees from Roche, GlaxoSmithKline, Glenmark and Medimmune, and research funding from UCB. Other authors have declared no competing interests.
Figures

Comment in
-
Standardisation of labial salivary gland biopsies in Sjogren's syndrome: importance for the practicing rheumatologist.Ann Rheum Dis. 2017 Jul;76(7):1159-1160. doi: 10.1136/annrheumdis-2016-210851. Epub 2017 Mar 2. Ann Rheum Dis. 2017. PMID: 28254788 No abstract available.
References
-
- Bowman SJ, Fox RI. Classification criteria for Sjogren's syndrome: nothing ever stands still!. Ann Rheum Dis 2014;73:1–2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

