Bone Marrow Cell Trafficking Analyzed by 89Zr-oxine Positron Emission Tomography in a Murine Transplantation Model
- PMID: 27965305
- PMCID: PMC5457332
- DOI: 10.1158/1078-0432.CCR-16-1561
Bone Marrow Cell Trafficking Analyzed by 89Zr-oxine Positron Emission Tomography in a Murine Transplantation Model
Abstract
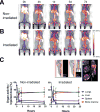
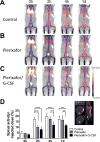
Purpose: The success of hematopoietic stem cell transplantation (HSCT) depends on donor cell homing to the bone marrow. However, there is no reliable method of noninvasively monitoring the kinetics and distribution of transferred cells. Using zirconium-89 (89Zr)-oxine cell labeling combined with PET imaging, we sought to visualize and quantify donor cell homing in a mouse bone marrow transplantation model.Experimental Design: The effect of 89Zr-oxine labeling on bone marrow cell viability and differentiation was evaluated in vitro89Zr-labeled bone marrow cells (2 × 107 cells, 16.6 kBq/106 cells) were transferred intravenously, and serial microPET images were obtained (n = 5). The effect of a CXCR4 inhibitor, plerixafor (5 mg/kg) and G-CSF (2.5 μg) on bone marrow homing and mobilization were examined (n = 4). Engraftment of the transferred 89Zr-labeled cells was evaluated (n = 3).Results:89Zr-oxine-labeled bone marrow cells showed delayed proliferation, but differentiated normally. Transferred bone marrow cells rapidly migrated to the bone marrow, spleen, and liver (n = 5). Approximately 36% of donor cells homed to the bone marrow within 4 hours, irrespective of prior bone marrow ablation. Inhibition of CXCR4 by plerixafor alone or with G-CSF significantly blocked the bone marrow homing (P < 0.0001, vs. nontreated, at 2 hours), confirming a crucial role of the CXCR4-CXCL12 system. Mobilization of approximately 0.64% of pretransplanted bone marrow cells induced a 3.8-fold increase of circulating bone marrow cells. 89Zr-labeled donor cells engrafted as well as nonlabeled cells.Conclusions:89Zr-oxine PET imaging reveals rapid bone marrow homing of transferred bone marrow cells without impairment of their stem cell functions, and thus, could provide useful information for optimizing HSCT. Clin Cancer Res; 23(11); 2759-68. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures




Similar articles
-
Bone marrow cell homing to sites of acute tibial fracture: 89Zr-oxine cell labeling with positron emission tomographic imaging in a mouse model.EJNMMI Res. 2018 Dec 13;8(1):109. doi: 10.1186/s13550-018-0463-8. EJNMMI Res. 2018. PMID: 30547233 Free PMC article.
-
(89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapies.Radiology. 2015 May;275(2):490-500. doi: 10.1148/radiol.15142849. Epub 2015 Feb 20. Radiology. 2015. PMID: 25706654 Free PMC article.
-
In Vivo Tracking of Adoptively Transferred Natural Killer Cells in Rhesus Macaques Using 89Zirconium-Oxine Cell Labeling and PET Imaging.Clin Cancer Res. 2020 Jun 1;26(11):2573-2581. doi: 10.1158/1078-0432.CCR-19-2897. Epub 2020 Feb 7. Clin Cancer Res. 2020. PMID: 32034075 Free PMC article.
-
Imaging of cell-based therapy using 89Zr-oxine ex vivo cell labeling for positron emission tomography.Nanotheranostics. 2021 Jan 1;5(1):27-35. doi: 10.7150/ntno.51391. eCollection 2021. Nanotheranostics. 2021. PMID: 33391973 Free PMC article. Review.
-
Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Clin Ther. 2010 May;32(5):821-43. doi: 10.1016/j.clinthera.2010.05.007. Clin Ther. 2010. PMID: 20685493 Review.
Cited by
-
Comparative strategies for stem cell biodistribution in a preclinical study.Acta Pharmacol Sin. 2020 Apr;41(4):572-580. doi: 10.1038/s41401-019-0313-x. Epub 2019 Nov 8. Acta Pharmacol Sin. 2020. PMID: 31705124 Free PMC article.
-
Evidence of Accumulated Endothelial Progenitor Cells in the Lungs of Rats with Pulmonary Arterial Hypertension by 89Zr-oxine PET Imaging.Mol Ther Methods Clin Dev. 2020 May 3;17:1108-1117. doi: 10.1016/j.omtm.2020.04.021. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32490032 Free PMC article.
-
In vivo tracking of ex-vivo-generated 89Zr-oxine-labeled plasma cells by PET in a non-human primate model.Mol Ther. 2025 Feb 5;33(2):580-594. doi: 10.1016/j.ymthe.2024.12.042. Epub 2024 Dec 30. Mol Ther. 2025. PMID: 39741408
-
Optimisation of the Synthesis and Cell Labelling Conditions for [89Zr]Zr-oxine and [89Zr]Zr-DFO-NCS: a Direct In Vitro Comparison in Cell Types with Distinct Therapeutic Applications.Mol Imaging Biol. 2021 Dec;23(6):952-962. doi: 10.1007/s11307-021-01622-z. Epub 2021 Jul 6. Mol Imaging Biol. 2021. PMID: 34231103 Free PMC article.
-
A kit formulation for the preparation of [89Zr]Zr(oxinate)4 for PET cell tracking: White blood cell labelling and comparison with [111In]In(oxinate)3.Nucl Med Biol. 2020 Nov-Dec;90-91:31-40. doi: 10.1016/j.nucmedbio.2020.09.002. Epub 2020 Sep 15. Nucl Med Biol. 2020. PMID: 32979725 Free PMC article.
References
-
- Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10:213–21. - PubMed
-
- Ramirez PA, Wagner JE, Brunstein CG. Going straight to the point: intra-BM injection of hematopoietic progenitors. Bone Marrow Transplant. 2010;45:1127–33. - PubMed
-
- Castello S, Podesta M, Menditto VG, Ibatici A, Pitto A, Figari O, et al. Intra-bone marrow injection of bone marrow and cord blood cells: an alternative way of transplantation associated with a higher seeding efficiency. Exp Hematol. 2004;32:782–7. - PubMed
-
- Frassoni F, Gualandi F, Podesta M, Raiola AM, Ibatici A, Piaggio G, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

