Involvement of c-Fos in the Promotion of Cancer Stem-like Cell Properties in Head and Neck Squamous Cell Carcinoma
- PMID: 27965308
- PMCID: PMC5468504
- DOI: 10.1158/1078-0432.CCR-16-2811
Involvement of c-Fos in the Promotion of Cancer Stem-like Cell Properties in Head and Neck Squamous Cell Carcinoma
Abstract
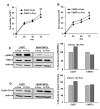
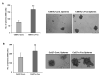
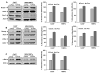
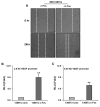
Purpose: Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide. Although improvements in surgical techniques, chemotherapy and radiation delivery, and supportive care have improved quality of life for patients with HNSCC, regional and distant recurrence remain common. Recent evidence suggests that cancer stem-like cells (CSC) play a significant role in recurrence and chemoresistance. We previously observed that c-Fos was highly upregulated in the HNSCC sphere-forming cells. Consequences of c-Fos upregulation for the biology of HNSCC-CSCs are poorly understood. In this study, we investigated the role of c-Fos in renewal of stemness of HNSCC and tumor growth.Experimental Design and Results: We generated stable HNSCC cell lines ectopically expressing the c-Fos gene. Exogenous expression of c-Fos in nontumorigenic MDA1386Tu cells makes these cells tumorigenic in nude mice. Furthermore, subcutaneous transplantation of c-Fos-overexpressing Cal27 cells (tumorigenic) into immunocompromised mice enhanced tumor growth as compared with parental cells. Mechanistic investigations demonstrated that c-Fos overexpression enhanced the epithelial-mesenchymal transition (EMT) state and expression of CSC markers (Nanog, c-Myc, Sox2, and Notch1). Ectopic expression of c-Fos in HNSCC cells also displays increased sphere formation. We further observed that overexpression of c-Fos increased the expression of pERK and cyclin D1 in HNSCC cells.Conclusions: Together, our results strongly suggest a novel role of c-Fos as a regulator of EMT and cancer stem cell reprogramming in HNSCC cells, which may hold potential as a CSC-directed therapeutic approach to improve HNSCC treatment. Clin Cancer Res; 23(12); 3120-8. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
MicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitro.Int J Oncol. 2015 Oct;47(4):1339-50. doi: 10.3892/ijo.2015.3142. Epub 2015 Aug 31. Int J Oncol. 2015. PMID: 26323460
-
Notch1 signaling contributes to stemness in head and neck squamous cell carcinoma.Lab Invest. 2016 May;96(5):508-16. doi: 10.1038/labinvest.2015.163. Epub 2016 Feb 29. Lab Invest. 2016. PMID: 26927514 Amharic, English.
-
5T4-Targeted Therapy Ablates Cancer Stem Cells and Prevents Recurrence of Head and Neck Squamous Cell Carcinoma.Clin Cancer Res. 2017 May 15;23(10):2516-2527. doi: 10.1158/1078-0432.CCR-16-1834. Epub 2016 Oct 25. Clin Cancer Res. 2017. PMID: 27780858 Free PMC article.
-
C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell.Oral Oncol. 2014 Jul;50(7):633-9. doi: 10.1016/j.oraloncology.2014.04.004. Epub 2014 May 15. Oral Oncol. 2014. PMID: 24835851 Review.
-
Epithelial-to-mesenchymal transition and cancer stem(-like) cells in head and neck squamous cell carcinoma.Cancer Lett. 2013 Sep 10;338(1):47-56. doi: 10.1016/j.canlet.2012.06.013. Epub 2012 Jul 4. Cancer Lett. 2013. PMID: 22771535 Review.
Cited by
-
Immune Escape Mechanisms and Their Clinical Relevance in Head and Neck Squamous Cell Carcinoma.Int J Mol Sci. 2020 Sep 24;21(19):7032. doi: 10.3390/ijms21197032. Int J Mol Sci. 2020. PMID: 32987799 Free PMC article. Review.
-
Targeting the Formyl Peptide Receptor type 1 to prevent the adhesion of ovarian cancer cells onto mesothelium and subsequent invasion.J Exp Clin Cancer Res. 2019 Nov 8;38(1):459. doi: 10.1186/s13046-019-1465-8. J Exp Clin Cancer Res. 2019. PMID: 31703596 Free PMC article.
-
c-Myb promotes growth and metastasis of colorectal cancer through c-fos-induced epithelial-mesenchymal transition.Cancer Sci. 2019 Oct;110(10):3183-3196. doi: 10.1111/cas.14141. Epub 2019 Aug 13. Cancer Sci. 2019. PMID: 31338937 Free PMC article.
-
Role of PCIF1-mediated 5'-cap N6-methyladeonsine mRNA methylation in colorectal cancer and anti-PD-1 immunotherapy.EMBO J. 2023 Jan 16;42(2):e111673. doi: 10.15252/embj.2022111673. Epub 2022 Dec 14. EMBO J. 2023. PMID: 36514940 Free PMC article.
-
Identification of the xenograft and its ascendant sphere-forming cell line as belonging to EBV-induced lymphoma, and characterization of the status of sphere-forming cells.Cancer Cell Int. 2019 May 6;19:120. doi: 10.1186/s12935-019-0842-x. eCollection 2019. Cancer Cell Int. 2019. PMID: 31080361 Free PMC article.
References
-
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. - PubMed
-
- Bakin AV, Curran T. Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science. 1999;283:387–390. - PubMed
-
- Dong C, Ye DX, Zhang WB, Pan HY, Zhang ZY, Zhang L. Overexpression of c-fos promotes cell invasion and migration via CD44 pathway in oral squamous cell carcinoma. J Oral Pathol Med. 2015;44:353–60. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

