Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice
- PMID: 27965322
- PMCID: PMC5295727
- DOI: 10.1096/fj.201600918R
Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice
Abstract
Articular cartilage has little regenerative capacity. Recently, genetic lineage tracing experiments have revealed chondrocyte progenitors at the articular surface. We further characterized these progenitors by using in vivo genetic approaches. Histone H2B-green fluorescent protein retention revealed that superficial cells divide more slowly than underlying articular chondrocytes. Clonal genetic tracing combined with immunohistochemistry revealed that superficial cells renew their number by symmetric division, express mesenchymal stem cell markers, and generate chondrocytes via both asymmetric and symmetric differentiation. Quantitative analysis of cellular kinetics, in combination with phosphotungstic acid-enhanced micro-computed tomography, showed that superficial cells generate chondrocytes and contribute to the growth and reshaping of articular cartilage. Furthermore, we found that cartilage renewal occurs as the progeny of superficial cells fully replace fetal chondrocytes during early postnatal life. Thus, superficial cells are self-renewing progenitors that are capable of maintaining their own population and fulfilling criteria of unipotent adult stem cells. Furthermore, the progeny of these cells reconstitute adult articular cartilage de novo, entirely substituting fetal chondrocytes.-Li, L., Newton, P. T., Bouderlique, T., Sejnohova, M., Zikmund, T., Kozhemyakina, E., Xie, M., Krivanek, J., Kaiser, J., Qian, H., Dyachuk, V., Lassar, A. B., Warman, M. L., Barenius, B., Adameyko, I., Chagin, A. S. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice.
Keywords: adult stem cells; bone; osteoarthritis; regeneration; superficial zone.
© FASEB.
Figures








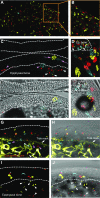
References
-
- Madry H., Luyten F. P., Facchini A. (2012) Biological aspects of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 20, 407–422 - PubMed
-
- Hall B. K. (2015) Bones and Cartilage: Developmental and Evolutionary Skeletal Biology, 2nd ed., Elsevier, New York
-
- Murray C. J., Vos T., Lozano R., Naghavi M., Flaxman A. D., Michaud C., Ezzati M., Shibuya K., Salomon J. A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S. Y., Ali M. K., Alvarado M., Anderson H. R., Anderson L. M., Andrews K. G., Atkinson C., Baddour L. M., Bahalim A. N., Barker-Collo S., Barrero L. H., Bartels D. H., Basáñez M. G., Baxter A., Bell M. L., Benjamin E. J., Bennett D., Bernabé E., Bhalla K., Bhandari B., Bikbov B., Bin Abdulhak A., Birbeck G., Black J. A., Blencowe H., Blore J. D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T. S., Bryan-Hancock C., Bucello C., Buchbinder R., Buckle G., Budke C. M., Burch M., Burney P., Burstein R., Calabria B., Campbell B., Canter C. E., Carabin H., Carapetis J., Carmona L., Cella C., Charlson F., Chen H., Cheng A. T., Chou D., Chugh S. S., Coffeng L. E., Colan S. D., Colquhoun S., Colson K. E., Condon J., Connor M. D., Cooper L. T., Corriere M., Cortinovis M., de Vaccaro K. C., Couser W., Cowie B. C., Criqui M. H., Cross M., Dabhadkar K. C., Dahiya M., Dahodwala N., Damsere-Derry J., Danaei G., Davis A., De Leo D., Degenhardt L., Dellavalle R., Delossantos A., Denenberg J., Derrett S., Des Jarlais D. C., Dharmaratne S. D., Dherani M., Diaz-Torne C., Dolk H., Dorsey E. R., Driscoll T., Duber H., Ebel B., Edmond K., Elbaz A., Ali S. E., Erskine H., Erwin P. J., Espindola P., Ewoigbokhan S. E., Farzadfar F., Feigin V., Felson D. T., Ferrari A., Ferri C. P., Fèvre E. M., Finucane M. M., Flaxman S., Flood L., Foreman K., Forouzanfar M. H., Fowkes F. G., Fransen M., Freeman M. K., Gabbe B. J., Gabriel S. E., Gakidou E., Ganatra H. A., Garcia B., Gaspari F., Gillum R. F., Gmel G., Gonzalez-Medina D., Gosselin R., Grainger R., Grant B., Groeger J., Guillemin F., Gunnell D., Gupta R., Haagsma J., Hagan H., Halasa Y. A., Hall W., Haring D., Haro J. M., Harrison J. E., Havmoeller R., Hay R. J., Higashi H., Hill C., Hoen B., Hoffman H., Hotez P. J., Hoy D., Huang J. J., Ibeanusi S. E., Jacobsen K. H., James S. L., Jarvis D., Jasrasaria R., Jayaraman S., Johns N., Jonas J. B., Karthikeyan G., Kassebaum N., Kawakami N., Keren A., Khoo J. P., King C. H., Knowlton L. M., Kobusingye O., Koranteng A., Krishnamurthi R., Laden F., Lalloo R., Laslett L. L., Lathlean T., Leasher J. L., Lee Y. Y., Leigh J., Levinson D., Lim S. S., Limb E., Lin J. K., Lipnick M., Lipshultz S. E., Liu W., Loane M., Ohno S. L., Lyons R., Mabweijano J., MacIntyre M. F., Malekzadeh R., Mallinger L., Manivannan S., Marcenes W., March L., Margolis D. J., Marks G. B., Marks R., Matsumori A., Matzopoulos R., Mayosi B. M., McAnulty J. H., McDermott M. M., McGill N., McGrath J., Medina-Mora M. E., Meltzer M., Mensah G. A., Merriman T. R., Meyer A. C., Miglioli V., Miller M., Miller T. R., Mitchell P. B., Mock C., Mocumbi A. O., Moffitt T. E., Mokdad A. A., Monasta L., Montico M., Moradi-Lakeh M., Moran A., Morawska L., Mori R., Murdoch M. E., Mwaniki M. K., Naidoo K., Nair M. N., Naldi L., Narayan K. M., Nelson P. K., Nelson R. G., Nevitt M. C., Newton C. R., Nolte S., Norman P., Norman R., O’Donnell M., O’Hanlon S., Olives C., Omer S. B., Ortblad K., Osborne R., Ozgediz D., Page A., Pahari B., Pandian J. D., Rivero A. P., Patten S. B., Pearce N., Padilla R. P., Perez-Ruiz F., Perico N., Pesudovs K., Phillips D., Phillips M. R., Pierce K., Pion S., Polanczyk G. V., Polinder S., Pope C. A. III, Popova S., Porrini E., Pourmalek F., Prince M., Pullan R. L., Ramaiah K. D., Ranganathan D., Razavi H., Regan M., Rehm J. T., Rein D. B., Remuzzi G., Richardson K., Rivara F. P., Roberts T., Robinson C., De Leòn F. R., Ronfani L., Room R., Rosenfeld L. C., Rushton L., Sacco R. L., Saha S., Sampson U., Sanchez-Riera L., Sanman E., Schwebel D. C., Scott J. G., Segui-Gomez M., Shahraz S., Shepard D. S., Shin H., Shivakoti R., Singh D., Singh G. M., Singh J. A., Singleton J., Sleet D. A., Sliwa K., Smith E., Smith J. L., Stapelberg N. J., Steer A., Steiner T., Stolk W. A., Stovner L. J., Sudfeld C., Syed S., Tamburlini G., Tavakkoli M., Taylor H. R., Taylor J. A., Taylor W. J., Thomas B., Thomson W. M., Thurston G. D., Tleyjeh I. M., Tonelli M., Towbin J. A., Truelsen T., Tsilimbaris M. K., Ubeda C., Undurraga E. A., van der Werf M. J., van Os J., Vavilala M. S., Venketasubramanian N., Wang M., Wang W., Watt K., Weatherall D. J., Weinstock M. A., Weintraub R., Weisskopf M. G., Weissman M. M., White R. A., Whiteford H., Wiebe N., Wiersma S. T., Wilkinson J. D., Williams H. C., Williams S. R., Witt E., Wolfe F., Woolf A. D., Wulf S., Yeh P. H., Zaidi A. K., Zheng Z. J., Zonies D., Lopez A. D., AlMazroa M. A., Memish Z. A. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

