PMS1T, producing phased small-interfering RNAs, regulates photoperiod-sensitive male sterility in rice
- PMID: 27965387
- PMCID: PMC5206514
- DOI: 10.1073/pnas.1619159114
PMS1T, producing phased small-interfering RNAs, regulates photoperiod-sensitive male sterility in rice
Abstract
Phased small-interfering RNAs (phasiRNAs) are a special class of small RNAs, which are generated in 21- or 24-nt intervals from transcripts of precursor RNAs. Although phasiRNAs have been found in a range of organisms, their biological functions in plants have yet to be uncovered. Here we show that phasiRNAs generated by the photopheriod-sensetive genic male sterility 1 (Pms1) locus were associated with photoperiod-sensitive male sterility (PSMS) in rice, a germplasm that started the two-line hybrid rice breeding. The Pms1 locus encodes a long-noncoding RNA PMS1T that was preferentially expressed in young panicles. PMS1T was targeted by miR2118 to produce 21-nt phasiRNAs that preferentially accumulated in the PSMS line under long-day conditions. A single nucleotide polymorphism in PMS1T nearby the miR2118 recognition site was critical for fertility change, likely leading to differential accumulation of the phasiRNAs. This result suggested possible roles of phasiRNAs in reproductive development of rice, demonstrating the potential importance of this RNA class as regulators in biological processes.
Keywords: long-noncoding RNA; phasiRNA; photoperiod-sensitive male sterility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




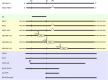



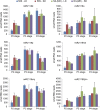


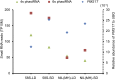


References
-
- Komiya R, et al. Rice germline-specific Argonaute MEL1 protein binds to phasiRNAs generated from more than 700 lincRNAs. Plant J. 2014;78(3):385–397. - PubMed
-
- Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121(2):207–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

